IL-6 trans-signaling mediates cytokine secretion and barrier dysfunction in hantavirus-infected cells and correlates to severity in HFRS
- PMID: 40203030
- PMCID: PMC12054857
- DOI: 10.1371/journal.ppat.1013042
IL-6 trans-signaling mediates cytokine secretion and barrier dysfunction in hantavirus-infected cells and correlates to severity in HFRS
Abstract
Background: Hantavirus causes hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS). Strong inflammatory responses and vascular leakage are important hallmarks of these often fatal diseases. The mechanism behind pathogenesis is unknown and no specific treatment is available. IL-6 was recently highlighted as a biomarker for HPS/HFRS severity. IL-6 signaling is complex and context dependent: while classical signaling generally provide protective responses, trans-signaling can cause severe pathogenic responses. Here, we investigated a potential role for IL-6 trans-signaling in hantavirus pathogenesis.
Methods: Effects of IL-6 trans-signaling during in vitro hantavirus infection were assessed using primary human endothelial cells treated with recombinant soluble IL-6 receptor (sIL-6R). Plasma from Puumala orthohantavirus-infected HFRS patients (n=28) were analyzed for IL-6 trans-signaling potential and its associations to severity.
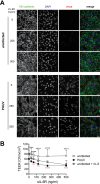
Findings: In vitro, sIL-6R treatment of infected cells enhanced IL-6 and CCL2 secretion, upregulated ICAM-1, and affected VE-cadherin leading to a disrupted cell barrier integrity. HFRS patients showed altered plasma levels of sIL-6R and soluble gp130 (sgp130) resulting in an increased sIL-6R/sgp130 ratio suggesting enhanced IL-6 trans-signaling potential. Plasma sgp130 levels negatively correlated with number of interventions and positively with albumin levels. Patients receiving oxygen treatment displayed a higher sIL-6R/sgp130 ratio compared to patients that did not.
Interpretation: IL-6 trans-signaling is linked to hantavirus pathogenesis. Targeting IL-6 trans-signaling might provide a therapeutic strategy for treatment of severe HFRS and perhaps also HPS.
Copyright: © 2025 Maleki et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Klingström J, Smed-Sörensen A, Maleki K, Solà-Riera C, Ahlm C, Björkström N. Innate and adaptive immune responses against human Puumala virus infection: immunopathogenesis and suggestions for novel treatment strategies for severe hantavirus-associated syndromes. J Inter Med. 2019;285:510–23. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

