Preclinical evaluation of AGT mRNA replacement therapy for primary hyperoxaluria type I disease
- PMID: 40203111
- PMCID: PMC11980851
- DOI: 10.1126/sciadv.adt9694
Preclinical evaluation of AGT mRNA replacement therapy for primary hyperoxaluria type I disease
Abstract
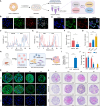
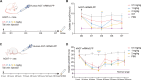
Primary hyperoxaluria type 1 (PH1) is a rare inherited liver disorder caused by alanine glyoxylate aminotransferase (AGT) dysfunction, leading to accumulation of glyoxylate which is then converted into oxalate. Excessive oxalate results in kidney damage due to deposition of oxalate crystals. We have developed an mRNA-based protein replacement therapy for PH1 to restore normal glyoxylate to glycine metabolism. Sequence optimized human AGT mRNA (hAGT mRNA) was encapsulated in lipopolyplex (LPP) and produced functional AGT enzyme in peroxisomes. Pharmacokinetics and pharmacodynamics (PK/PD) were evaluated in vitro and in vivo. PK demonstrated that AGT mRNA and AGT protein maintained high expression levels for up to 48 hours. A single 2 mg/kg dose in AgxtQ84-/- rats achieved a 70% reduction in urinary oxalate. Toxicological assessment identified the highest nonserious toxic dose (HNSTD) as 2 mg/kg. These findings affirm the efficacy and safety of hAGT mRNA/LPP and support its clinical application in PH1 treatment.
Figures





References
-
- Cochat P., Rumsby G., Primary hyperoxaluria. N. Engl. J. Med. 369, 649–658 (2013). - PubMed
-
- Danpure C. J., Molecular etiology of primary hyperoxaluria type 1: New directions for treatment. Am. J. Nephrol. 25, 303–310 (2005). - PubMed
-
- Cellini B., Oppici E., Paiardini A., Montioli R., Molecular insights into primary hyperoxaluria type 1 pathogenesis. Front. Biosci. 17, 621–634 (2012). - PubMed
-
- Fargue S., Rumsby G., Danpure C. J., Multiple mechanisms of action of pyridoxine in primary hyperoxaluria type 1. Biochim. Biophys. Acta 1832, 1776–1783 (2013). - PubMed
-
- Oppici E., Montioli R., Cellini B., Liver peroxisomal alanine:glyoxylate aminotransferase and the effects of mutations associated with primary hyperoxaluria type I: An overview. Biochim. Biophys. Acta 1854, 1212–1219 (2015). - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

