Chemoenzymatic Dynamic Kinetic Resolution of Atropoisomeric 2-(Quinolin-8-yl)benzylalcohols
- PMID: 40203203
- PMCID: PMC12160057
- DOI: 10.1021/acs.joc.4c02996
Chemoenzymatic Dynamic Kinetic Resolution of Atropoisomeric 2-(Quinolin-8-yl)benzylalcohols
Abstract
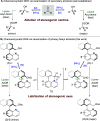
The chemoenzymatic dynamic kinetic resolution of 2-(quinolin-8-yl)benzylalcohols using a combination of lipases and ruthenium catalysts is described. While CalB lipase performs highly selective enzymatic kinetic resolution, the combination with Shvo's or Bäckvall's catalysts promotes atropisomerization of the substrate via the reversible formation of configurationally labile aldehydes, thereby enabling a dynamic kinetic resolution. This synergistic approach was applied to the synthesis of a variety of heterobiaryl acetates in excellent yields and enantioselectivities.
Figures



References
-
- Feng J., Liu R.-R.. Catalytic Asymmetric Synthesis of N–N Biaryl Atropisomers. Chem.Eur. J. 2024;30:e202303165. doi: 10.1002/chem.202303165. - DOI - PubMed
- Roos C. B., Chiang C.-H., Murray L. A. M., Yang D., Schulert L., Narayan A. R. H.. Stereodynamic Strategies to Induce and Enrich Chirality of Atropisomers at a Late Stage. Chem. Rev. 2023;123:10641–10727. doi: 10.1021/acs.chemrev.3c00327. - DOI - PubMed
- Rodríguez-Salamanca P., Fernández R., Hornillos V., Lassaletta J. M.. Asymmetric Synthesis of Axially Chiral C–N Atropisomers. Chem.Eur. J. 2022;28:e202104442. doi: 10.1002/chem.202104442. - DOI - PMC - PubMed
- Tan, B. Axially Chiral Compounds: Asymmetric Synthesis and Applications; Wiley-VCH, 2021.
- Carmona J. A., Rodríguez-Franco C., Fernández R., Hornillos V., Lassaletta J. M.. Atroposelective transformation of axially chiral (hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 2021;50:2968–2983. doi: 10.1039/D0CS00870B. - DOI - PubMed
- Da B.-C., Xiang S.-H., Li S., Tan B.. Chiral Phosphoric Acid Catalyzed Asymmetric Synthesis of Axially Chiral Compounds. Chin. J. Chem. 2021;39:1787–1796. doi: 10.1002/cjoc.202000751. - DOI
- Cheng J. K., Xiang S. H., Li S., Ye L., Tan B.. Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev. 2021;121:4805–4902. doi: 10.1021/acs.chemrev.0c01306. - DOI - PubMed
- Lassaletta, J. M. Atropisomerism and Axial Chirality; World Scientific, 2019.
-
- Overacker R. D., Banerjee S., Neuhaus G. F., Sephton S. M., Herrmann A., Strother J. A., Brack-Werner R., Blakemore P. R., Loesgen S.. Biological evaluation of molecules of the azaBINOL class as antiviral agents: Inhibition of HIV-1 RNase H activity by 7-isopropoxy-8-(naphth-1-yl) quinoline. Bioorg. Med. Chem. 2019;27:3595–3604. doi: 10.1016/j.bmc.2019.06.044. - DOI - PubMed
- Rokade B. V., Guiry P. J.. Axially Chiral P,N-Ligands: Some Recent Twists and Turns. ACS Catal. 2018;8:624–643. doi: 10.1021/acscatal.7b03759. - DOI
- Wu Z., Wang C., Zakharov L. N., Blakemore P. R.. Enantioselective Synthesis of Biaryl Compounds via Suzuki–Miyaura Cross-Coupling Using a Palladium Complex of 7′-Butoxy-7-(diphenylphosphino)-8,8′-biquinolyl: Investigation of a New Chiral Ligand Architecture. Synthesis. 2014;46:678–685. doi: 10.1055/s-0033-1340519. - DOI
-
- On I. K. W., Hong W., Zhu Y.. Crossing the ortho-hurdle: Ionic stereocontrol enables atroposelective Suzuki-Miyaura coupling. Chem. Catal. 2023;3:100523. doi: 10.1016/j.checat.2023.100523. - DOI
- Shen D., Xu Y., Shi S.-L.. A Bulky Chiral N-Heterocyclic Carbene Palladium Catalyst Enables Highly Enantioselective Suzuki–Miyaura Cross-Coupling Reactions for the Synthesis of Biaryl Atropisomers. J. Am. Chem. Soc. 2019;141:14938–14945. doi: 10.1021/jacs.9b08578. - DOI - PubMed
LinkOut - more resources
Full Text Sources

