Patient anti-FVIII drug antibodies bind preferentially to a subset of FVIII covalent states
- PMID: 40203231
- PMCID: PMC12305225
- DOI: 10.1182/bloodadvances.2025016474
Patient anti-FVIII drug antibodies bind preferentially to a subset of FVIII covalent states
Abstract
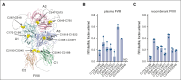
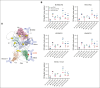
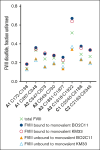
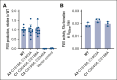
Hemophilia A is a chronic life-threatening condition caused by the deficiency or dysfunction of plasma coagulation factor VIII (FVIII) and commonly managed by prophylaxis with regular infusion of FVIII protein. A major obstacle to FVIII replacement therapy is the generation of alloantibodies that diminish efficacy. Disulfide bonds link pairs of cysteine residues in proteins and, in several proteins, have been found to be only partially formed in the mature proteins. FVIII contains 8 disulfide bonds and their redox state in human blood and recombinant FVIII was determined using differential cysteine alkylation and mass spectrometry. All 8 disulfide bonds were found to be unformed in ∼10% to ∼70% of molecules of FVIII populations, which suggested a conformational flexibility that could favor the binding of certain ligands to subsets of FVIII with more or less formed disulfide bonds. To test this hypothesis, the binding of a panel of 5 patient-derived anti-FVIII antibodies to the population of FVIII disulfide-bonded states was evaluated. All 5 antibodies bound preferentially to FVIII states in which 2 or 3 of the 8 disulfides are significantly more unformed: C1918-C1922 in the A3 domain, C2040-C2188 in the C1 domain, and C2193-C2345 in the C2 domain. Disulfide bond mutagenesis experiments and molecular dynamics simulations indicate that this subset of FVIII states has long-range conformational dynamism that favors antidrug antibody binding. These findings will assist efforts to engineer an FVIII molecule that is less prone to neutralization by antidrug antibodies and has general implications for autoimmune conditions and antibody drug efficacy.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



Comment in
-
Exorcizing the devil of factor VIII inhibitors.Blood Adv. 2025 Aug 12;9(15):3716-3717. doi: 10.1182/bloodadvances.2025016643. Blood Adv. 2025. PMID: 40705342 Free PMC article. No abstract available.
References
-
- Pipe SW, Gonen-Yaacovi G, Segurado OG. Hemophilia A gene therapy: current and next-generation approaches. Expert Opin Biol Ther. 2022;22(9):1099–1115. - PubMed
-
- Pipe SW. Bioengineered molecules for the management of haemophilia: promise and remaining challenges. Haemophilia. 2018;24(suppl 6):68–75. - PubMed
-
- Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta-analytic approach using national registries. Ann Intern Med. 2019;171(8):540–546. - PubMed
-
- Valentino LA, Khair K. Prophylaxis for hemophilia A without inhibitors: treatment options and considerations. Expert Rev Hematol. 2020;13(7):731–743. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

