Mathematical modeling unveils the timeline of CAR-T cell therapy and macrophage-mediated cytokine release syndrome
- PMID: 40203243
- PMCID: PMC11981663
- DOI: 10.1371/journal.pcbi.1012908
Mathematical modeling unveils the timeline of CAR-T cell therapy and macrophage-mediated cytokine release syndrome
Abstract
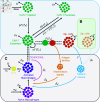
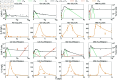
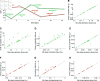
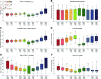
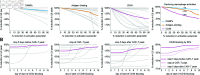
Chimeric antigen receptor (CAR)-T cell therapy holds significant potential for cancer treatment, although disease relapse and cytokine release syndrome (CRS) remain as frequent clinical challenges. To better understand the mechanisms underlying the temporal dynamics of CAR-T cell therapy response and CRS, we developed a novel multi-layer mathematical model incorporating antigen-mediated CAR-T cell expansion, antigen-negative resistance, and macrophage-associated cytokine release. Three key mechanisms of macrophage activation are considered: release of damage-associated molecular patterns, antigen-binding mediated activation, and CD40-CD40L contact. The model accurately describes 25 patient time courses with different responses and IL-6 cytokine kinetics. We successfully link the dynamic shape of the response to interpretable model parameters and investigate the influence of CAR-T cell dose and initial tumor burden on the occurrence of cytokine release and treatment outcome. By disentangling the timeline of macrophage activation, the model identified distinct contributions of each activation mechanism, suggesting the CD40-CD40L axis as a major driver of cytokine release and a clinically feasible target to control the activation process and modulate cytokine peak height. Our multi-layer model provides a comprehensive framework for understanding the complex interactions between CAR-T cells, tumor cells, and macrophages during therapy.
Copyright: © 2025 Santurio et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

