Comparative efficacy of Bruton tyrosine kinase inhibitors in high-risk relapsed/refractory CLL: a network meta-analysis
- PMID: 40203277
- PMCID: PMC12180973
- DOI: 10.1182/bloodadvances.2024014523
Comparative efficacy of Bruton tyrosine kinase inhibitors in high-risk relapsed/refractory CLL: a network meta-analysis
Abstract
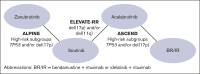
Bruton tyrosine kinase inhibitors (BTKis) have led to changes in the treatment algorithm for patients with high-risk relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL), defined based on the presence of genetic mutations. Given the lack of head-to-head trials comparing next-generation BTKis used to treat high-risk R/R disease, a network meta-analysis (NMA) was performed to estimate their relative efficacy. High-risk populations were defined based on the prespecified definitions within each trial, including patients with del(17p) and/or TP53 mutations in the ALPINE (n = 150) and ASCEND (n = 86) trials, and del(17p)/del(11q) in the ELEVATE-RR (n = 533) trial. Bayesian NMAs found zanubrutinib to be the most efficacious treatment for high-risk patients, with significantly reduced risk of progression or death compared with ibrutinib (hazard ratio [HR], 0.49; 95% credible interval [CrI], 0.31-0.78), acalabrutinib (HR, 0.55; 95% CrI, 0.32-0.94), and bendamustine + rituximab or idelalisib + rituximab (BR/IR; HR, 0.12; 95% CrI, 0.05-0.26). Differences in overall survival demonstrated a numerical trend favoring zanubrutinib (probability better than ≥80%) compared with ibrutinib (HR, 0.59; 95% CrI, 0.31-1.11), acalabrutinib (HR, 0.72; 95% CrI, 0.35-1.50), and BR/IR (HR, 0.65; 95% CrI, 0.23-1.75). Rates of response also demonstrated trends favoring zanubrutinib compared with acalabrutinib, with significant results compared with ibrutinib. The NMA suggests that the most efficacious BTKi for patients with high-risk R/R CLL is zanubrutinib.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.S. reports consulting role with, and serving on advisory boards, steering committees, or data safety monitoring committees for, AbbVie, Genentech, AstraZeneca, Genmab, Janssen, BeiGene, Bristol Myers Squibb, MorphoSys/Incyte, Kite Pharma, Eli Lilly, Mustang Bio, Fate therapeutics, Nurix, and Merck; reports institutional research funding from Mustang Bio, Genentech, AbbVie, BeOne Medicines Ltd, AstraZeneca, Genmab, MorphoSys/Incyte, and Vincerx; reports stock options with Koi Biotherapeutics; and reports employment with Bristol Myers Squibb (spouse). J.R.B. has served as a consultant for AbbVie, Acerta/AstraZeneca, Alloplex Biotherapeutics, BeOne Medicines Ltd, Bristol Myers Squibb, Galapagos NV, Genentech/Roche, Grifols Worldwide Operations, InnoCare Pharma Inc, iOnctura, Kite Pharma, Loxo/Lilly, Merck, Numab Therapeutics, Pfizer, and Pharmacyclics; received research funding from BeOne Medicines Ltd, Gilead, iOnctura, Loxo/Lilly, MEI Pharma, and TG Therapeutics; and serves on the data safety monitoring board for Grifols Therapeutics. L.M., K.Y., and R.W. are employees of BeOne Medicines Ltd and own stock in BeOne Medicines Ltd. H.B. is an employee of Evidera, a Thermo Fisher company, which provides consulting and other research services to life science companies; in her salaried positions, she works with a variety of companies and is precluded from receiving payment or honoraria directly from these organizations for services rendered; Evidera received payment from BeOne Medicines Ltd for the conduct of this study. B.N. is an employee of Evidera, a Thermo Fisher company, which provides consulting and other research services to life science companies; in his salaried positions, he works with a variety of companies and is precluded from receiving payment or honoraria directly from these organizations for services rendered; Evidera received payment from BeOne Medicines Ltd for the conduct of this study. N.L. reports scientific advisory board participation/consultants for/honoraria from AbbVie, Adaptive Biosciences, Allogene Therapeutics, AstraZeneca, BeiGene, Genentech, Janssen, Loxo/Eli Lilly, and Pharmacyclics; and reports honoraria from Aptitude Health, Bio Ascend, Clinical Care Options, Curio, DAVA Oncology, OncLive, Physicians' Education Resource, PeerView, and Targeted Oncology; and reports institutional research funding from AbbVie, AstraZeneca, BeiGene, Genentech, Genmab, Loxo/Eli Lilly, MingSight, Octapharma, and Oncternal. S.M.O. reports employment with University of California, Irvine; reports honoraria from Celgene, Janssen, Pharmacyclics, Gilead Sciences, Pfizer, Amgen, Astellas Pharma, GlaxoSmithKline, Aptose Biosciences, Vaniam Group, AbbVie, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Eisai, TG Therapeutics, and NOVA Research; reports consulting or advisory roles with Amgen, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences, Vaniam Group, AbbVie/Genentech, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Astellas Pharma, Gilead Sciences, Pharmacyclics, TG Therapeutics, Pfizer, and Sunesis Pharmaceuticals; received research funding from Acerta Pharma (institutional), Regeneron (institutional), Gilead Sciences (institutional), Pfizer (institutional), TG Therapeutics (institutional), Pharmacyclics (institutional), Kite (a Gilead company [institutional]), Sunesis Pharmaceuticals (institutional), Lilly (institutional), and Caribou Biosciences (institutional); and travel, accommodations, and expenses from Celgene, Janssen, Gilead Sciences, Regeneron, and Janssen Oncology. A.T. reports receiving consulting fees from AbbVie, AstraZeneca, BeOne Medicines Ltd, and Janssen, and reports honoraria from AbbVie, AstraZeneca, BeOne Medicines Ltd, and Janssen. C.S.T. reports receiving funding from Janssen-Cilag (institutional), AbbVie (institutional), and BeOne Medicines Ltd (institutional); reports consulting or advisory roles with Janssen, Loxo, Roche, BeiGene, and AbbVie; and received honoraria from Janssen-Cilag, AbbVie, Novartis, BeOne Medicines Ltd, Pharmacyclics, Roche/Genentech, and Loxo/Lilly.
Figures



References
-
- Shadman M. Diagnosis and treatment of chronic lymphocytic leukemia: a review. Jama. 2023;329(11):918–932. - PubMed
-
- NIH National Cancer Institute. Chronic lymphocytic leukemia treatment (PDQ)–health professional version. https://www.cancer.gov/types/leukemia/hp/cll-treatment-pdq - PubMed
-
- Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724–3734. - PubMed
-
- Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23–33. - PubMed
-
- Walewska R, Parry-Jones N, Eyre TA, et al. Guideline for the treatment of chronic lymphocytic leukaemia. Br J Haematol. 2022;197(5):544–557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

