Understanding Infection, Viral Exacerbation and Respiratory Symptoms at Admission-Longitudinal (UNIVERSAL) study: a prospective observational cohort study protocol
- PMID: 40204301
- PMCID: PMC11987089
- DOI: 10.1136/bmjopen-2024-093427
Understanding Infection, Viral Exacerbation and Respiratory Symptoms at Admission-Longitudinal (UNIVERSAL) study: a prospective observational cohort study protocol
Abstract
Background: Respiratory viral infections (RVIs) are a significant cause of morbidity and hospital admission worldwide. However, the management of most viral infection-associated diseases remains primarily supportive. The recent COVID-19 pandemic has underscored the urgent need for a deeper understanding of RVIs to improve patient outcomes and develop effective treatment strategies. The Understanding Infection, Viral Exacerbation and Respiratory Symptoms at Admission-Longitudinal Study is an observational study which addresses this need by investigating the heterogeneity of RVIs in hospitalised adults, aiming to identify clinical and biological predictors of adverse outcomes. This study aims to bridge critical knowledge gaps in the clinical course and the economic impact of RVIs by characterising the phenotypic diversity of these infections and their recovery patterns following hospital admission and thus assisting with the optimal design of future interventional studies.
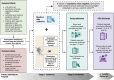
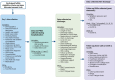
Methods and analysis: This prospective longitudinal observational study (V.6, 20 September 2023) will be conducted across multiple UK secondary care sites from August 2022 onwards, with an aim to enrol 1000 participants testing positive for RVI. Adults admitted with respiratory symptoms who test positive for RVIs via the BioFire® FilmArray® System or other validated diagnostic PCR tests will be enrolled. The data collected include patient demographics, clinical history, comorbidities and symptoms experienced prior to, during and after hospitalisation with follow-up after discharge at weeks 1, 2, 4, 8, 12 and 26. In addition, biological samples are collected at multiple time points during the hospital stay. The primary endpoints are to study the impact of different RVIs and identify predictors of disease progression and length of stay. Secondary endpoints include time to recovery and healthcare cost. Exploratory endpoints focus on biomarker profiles associated with virus type and clinical outcomes.
Ethics and dissemination: The study protocol received ethical approval from the relevant committees (English Ethics Reference Number: 22/WM/0119; Scottish Ethics Reference Number: 22-SS-0101, 20/09/2023). For patients who lack the capacity to consent, the study complies with the Mental Capacity Act 2005, using a consultee process where a family member, carer or an independent clinician may provide assent on behalf of the patient. Data from all the study centres will be analysed together and disseminated through peer-reviewed journals, conference presentations and workshops. The study group will ensure that participants and their families are informed of the study findings promptly and in an accessible format.
Trial registration number: ISRCTN49183956.
Keywords: Hospitalization; Observational Study; Patient Reported Outcome Measures; Respiratory infections; SARS-CoV-2 Infection.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: PR has options in Synairgen plc. BC is an employee of Synairgen Research Ltd and has options in Synairgen plc. SA and SD are employees of Synairgen Research Ltd and have options and shares in Synairgen plc. SS has received fees for advisory services/speaker fees from Astra Zeneca, Chiesi, GSK, Areteia therapeutics, CSL Behring, Medscape. KJS reports grants from AstraZeneca and Epiendo and speakers’ honoraria from AstraZeneca. TWC has received research grants from BioFire diagnostics, Biomerieux, QIAGEN, Sense Biodetection and Inflammatix. He has received speaker fees, honoraria and travel re-imbursement from BioFire diagnostics, BioMerieux, QIAGEN, Cepheid and Janssen. He has received consultancy fees from BioMerieux, QIAGEN, Cepheid, Roche, Janssen and Synairgen and has been a member of advisory boards for Cepheid, Roche, Roche diagnostics, Janssen, GSK, Shiongi, Sanofi and Seqirus. He is a member of an independent data monitoring committee for a trial sponsored by Roche. He owns shares in Synairgen plc. TW has received grants and fees from AstraZeneca, Bergenbio, Boehringer Ingelheim, Chiesi, GSK, Janssen, Olam, MMH, Synairgen, Union Chimique Belge and Valneva.
Figures
References
-
- Dietz E, Pritchard E, Pouwels K, et al. SARS-CoV-2, influenza A/B and respiratory syncytial virus positivity and association with influenza-like illness and self-reported symptoms, over the 2022/23 winter season in the UK: a longitudinal surveillance cohort. BMC Med. 2024;22:143. doi: 10.1186/s12916-024-03351-w. - DOI - PMC - PubMed
-
- Zhou F, Wang Y, Liu Y, et al. Disease severity and clinical outcomes of community-acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicentre prospective registry study from the CAP-China Network. Eur Respir J. 2019;54:1802406. doi: 10.1183/13993003.02406-2018. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
