Clinical predictors of flare and drug-free remission in rheumatoid arthritis: preliminary results from the prospective BIO-FLARE experimental medicine study
- PMID: 40204305
- PMCID: PMC11987156
- DOI: 10.1136/bmjopen-2024-092478
Clinical predictors of flare and drug-free remission in rheumatoid arthritis: preliminary results from the prospective BIO-FLARE experimental medicine study
Abstract
Objectives: Huge advances in rheumatoid arthritis (RA) treatment mean an increasing number of patients now achieve disease remission. However, long-term treatments can carry side effects and associated financial costs. In addition, some patients still experience painful and debilitating disease flares, the mechanisms of which are poorly understood. High rates of flare and a lack of effective prediction tools can limit attempts at treatment withdrawal. The BIOlogical Factors that Limit sustAined Remission in rhEumatoid arthritis (BIO-FLARE) experimental medicine study was designed to study flare and remission immunobiology. Here, we present the clinical outcomes and predictors of drug-free remission and flare, and develop a prediction model to estimate flare risk.
Design, setting and participants: BIO-FLARE was a multicentre, prospective, single-arm, open-label experimental medicine study conducted across seven National Health Service Trusts in the UK. Participants had established RA in clinical remission (disease activity score in 28 joints with C reactive protein (DAS28-CRP)<2.4) and were receiving methotrexate, sulfasalazine or hydroxychloroquine (monotherapy or combination).
Interventions: The intervention was disease-modifying anti-rheumatic drug cessation, followed by observation for 24 weeks or until flare, with clinical and immune monitoring.
Outcome measures: The primary outcome measure was the proportion of participants experiencing a confirmed flare, defined as DAS28-CRP≥3.2 or DAS28-CRP≥2.4 twice within 2 weeks, and time to flare. Exploratory predictive modelling was also performed using multivariable Cox regression to understand risk factors for flare.
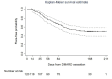
Results: 121 participants were recruited between September 2018 and December 2020. Flare rate by week 24 was 52.3% (95% CI 43.0 to 61.7), with a median (IQR) time to flare of 63 (41-96) days. Female sex, baseline methotrexate use, anti-citrullinated peptide antibody level and rheumatoid factor level were associated with flare. An exploratory prediction model incorporating these variables allowed estimation of flare risk, with acceptable classification (C index 0.709) and good calibration performance.
Conclusion: The rate of flare was approximately 50%. Several baseline clinical parameters were associated with flare. The BIO-FLARE study design provides a robust experimental medicine model for studying flare and remission immunobiology.
Trial registration number: ISRCTN registry 16371380.
Keywords: Drug Therapy; IMMUNOLOGY; Rheumatology.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: JI discloses research grants from Pfizer, Janssen and GSK; conference support from Eli Lilly; and has consulted for Abbvie, Anaptys Bio, AstraZeneca, BMS, Eli Lilly, Galapagos NV, Gilead Sciences Ltd, GSK, Istesso Ltd, Kira Biotech, Ono Pharma, Pfizer, Revelo Biotherapeutics, Roche and Sanofi. SS reports institutional research grants from Amgen (previously Celgene), Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, GSK, Janssen and UCB; speaker/consulting fees from AbbVie, Amgen, AstraZeneca, Eli Lilly, GSK, Janssen and UCB. IBM declares honoraria or research support from Abbvie, Janssen, Novartis, Eli Lilly, Astra Zeneca, GSK, BMS, Moonlake, Evelo, Causeway THerapeutics, Cabaletta, Roche, Pfizer and Compugen. CMUH declares research funding from GSK. KFB declares research support from Genentech, clinical improvement funding from Pfizer and consulting fees from Modern Biosciences. AP, KFB and JI are named as inventors on a patent application by Newcastle University ('Prediction of Drug-Free Remission in Rheumatoid Arthritis'; International Patent Application Number PCT/GB2019/050902). KR has received research grant support from Bristol Myers Squibb and personal fees for lectures/consultancy from Abbvie and Sanofi. AF reports institutional research grants from BMS, Roche, UCB, Nascient, Mestag, GSK, Janssen and speaker/consulting fees from Roche, Janssen and Sonoma.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
