SMALL: open surgery versus minimally invasive vacuum-assisted excision for small screen-detected breast cancer-protocol for a phase III randomised multicentre trial
- PMID: 40204306
- PMCID: PMC11979497
- DOI: 10.1136/bmjopen-2025-099702
SMALL: open surgery versus minimally invasive vacuum-assisted excision for small screen-detected breast cancer-protocol for a phase III randomised multicentre trial
Abstract
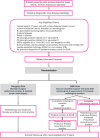
Introduction: Mammographic screening identifies many women with small breast cancers with favourable biological features, which have an excellent prognosis. Some of these may never have become clinically apparent without screening and are commonly described as 'overdiagnosed' cancers. Despite this, all patients with screen-detected cancers are currently treated with surgical excision and sentinel lymph node biopsy, although this may represent overtreatment. There is, therefore, a need for less invasive approaches to reduce treatment burden for patients while maintaining current excellent oncological outcomes. Vacuum-assisted excision (VAE) may represent such an alternative treatment approach, and the SMALL (Open Surgery versus Minimally invasive-vacuum Assisted excision for smaLL screen-detected breast cancer) trial aims to investigate the use of VAE for the safe de-escalation of surgical treatment for such excellent prognosis invasive breast cancers.
Methods: SMALL is a prospective, multicentre, randomised phase III trial of VAE versus surgery in patients with small, biologically favourable screen-detected invasive breast cancer. SMALL has an innovative hybrid design with coprimary endpoints. These include a randomised non-inferiority comparison of surgical re-excision rates following initial treatment, and a single-arm analysis of local recurrence at 5 years following VAE. Secondary outcomes include complication rates, overall survival, quality of life and a health economic analysis. The trial includes a QuinteT Recruitment Intervention to support recruitment.
Ethics and dissemination: Ethical approval was obtained from the Office for Research Ethics (Northern Ireland) for all UK sites. Results will be submitted for publication in a peer-reviewed journal, presented, shared with patient partners and with relevant professional organisations to inform future guideline development for the management of screen-detected breast cancer.
Trial registration number: ISRCTN12240119.
Keywords: Breast imaging; Breast surgery; Breast tumours; Clinical Trial.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: SM reports speaker honoraria from MSD, Roche, BD, Astra Zeneca, Veracyte and Exact Sciences; advisory boards for Lilly, Novartis, MSD, Roche and Astra Zeneca; conference travel and support from Roche, Lilly and MSD, and institutional research funding from Novartis. S Pinder reports honoraria from Roche, Astra Zeneca, Exact Sciences and Daiichi-Sankyo; advisory board for Exact Science. NS reports speaker honoraria from BD and Hologic. The remaining authors have no competing interests to declare.
Figures
References
-
- Narod SA, Iqbal J, Miller AB. Why have breast cancer mortality rates declined? J Cancer Policy. 2015;5:8–17. doi: 10.1016/j.jcpo.2015.03.002. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
