Genetic Risk and First-Trimester Cardiovascular Health Predict Hypertensive Disorders of Pregnancy in Nulliparous Women
- PMID: 40204378
- PMCID: PMC12042077
- DOI: 10.1016/j.jacc.2025.02.015
Genetic Risk and First-Trimester Cardiovascular Health Predict Hypertensive Disorders of Pregnancy in Nulliparous Women
Abstract
Background: Hypertensive disorders of pregnancy (HDPs) (preeclampsia/eclampsia and gestational hypertension) are a leading cause of maternal and perinatal morbidity and mortality and are associated with long-term maternal cardiovascular disease. High genetic risk and poor cardiovascular health (CVH) are each associated with HDPs, but whether genetic risk for HDP is modified by CVH status in early pregnancy is unknown.
Objectives: In this study, the authors sought to test the independent and joint associations of genetic risk and first-trimester CVH with development of HDP.
Methods: We examined genotyped participants from the nuMoM2b (Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be), a prospective observational cohort that enrolled nulliparous individuals with singleton pregnancies from 2010 to 2013 at 8 U.S. clinical sites. Genetic risk was calculated according to a validated genetic risk score for HDP. A first-trimester CVH score was closely adapted from the American Heart Association Life's Essential 8 model. Genetic risk and CVH were each categorized as low (bottom quintile), intermediate (quintile 2-4), or high (top quintile). The primary outcome was development of HDP. Multivariable-adjusted logistic regression was used to test the independent and joint associations of genetic risk and CVH with development of HDPs.
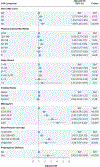
Results: Among 7,499 participants (mean age 27.0 years), the median first-trimester CVH score was 77.1 (Q1-Q3: 67.1-85.7). Overall, 1,032 participants (13.8%) developed an HDP (487 [6.5%] preeclampsia, 545 [7.3%] gestational hypertension). Genetic risk and CVH were each independently and additively associated with HDP (high vs low genetic risk: adjusted OR [aOR]: 2.21 [95% CI: 1.78-2.77; P < 0.001]; low vs high CVH: aOR: 2.92 [95% CI: 2.28-3.74; P < 0.001]). There was no significant interaction between genetic risk and CVH regarding risk of HDPs (Pinteraction > 0.05). HDP incidence ranged from 4.5% (low genetic risk, high CVH) to 25.7% (high genetic risk, low CVH). Compared with low CVH, high CVH was associated with 53%-74% lower risk of HDP across genetic risk strata. Findings were consistent when examining preeclampsia/eclampsia and gestational hypertension separately.
Conclusions: Lower genetic risk and higher first-trimester CVH were independently and additively associated with lower risk of developing HDPs in nulliparous individuals. Favorable CVH in early pregnancy may mitigate high genetic risk for HDP.
Keywords: cardiovascular health; hypertension; hypertensive disorders of pregnancy; polygenic risk scores; preventive cardiology.
Copyright © 2025 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Dr Honigberg is supported by the National Heart, Lung, and Blood Institute (NHLBI; K08HL166687), American Heart Association (940166, 24RGRSG1275749), and Patient-Centered Outcomes Research Institute. The nuMoM2b cohort was supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; U10 HD063036, U10 HD063072, U10 HD063047, U10 HD063037, U10 HD063041, U10 HD063020, U10 HD063046, U10 HD063048, and U10 HD063053) and the Clinical and Translational Science Institutes (UL1TR001108 and UL1TR000153). The nuMoM2b Heart Health Study was supported by cooperative agreement funding from the NHLBI and the NICHD (U10-HL119991; U10-HL119989; U10-HL120034; U10-HL119990; U10-HL120006; U10-HL119992; U10-HL120019; U10-HL119993; U10-HL120018, and U01HL145358), the National Center for Advancing Translational Sciences (UL-1-TR000124, UL-1-TR000153, UL-1-TR000439, and UL-1-TR001108), the Barbra Streisand Women’s Cardiovascular Research and Education Program, and the Erika J. Glazer Women’s Heart Research Initiative, Cedars-Sinai Medical Center, Los Angeles, with supplemental support for NHLBI U10-HL119991 from the Office of Research on Women’s Health and the Office of Disease Prevention. DNA extraction and genome-wide association studies of nuMoM2b specimens were supported by the Indiana University Grand Challenges Precision Diabetes Project. Dr Natarajan has received research grants from Allelica, Amgen, Apple, Boston Scientific, Genentech/Roche, and Novartis; has received personal fees from Allelica, Apple, AstraZeneca, Blackstone Life Sciences, Creative Education Concepts, CRISPR Therapeutics, Eli Lilly & Co, Foresite Labs, Genentech/Roche, GV, HeartFlow, Magnet Biomedicine, Merck, and Novartis; is a scientific advisory board member for Esperion Therapeutics, Preciseli, and TenSixteen Bio; is a scientific co-founder of TenSixteen Bio; has equity in MyOme, Preciseli, and TenSixteen Bio; and his spouse is employed by Vertex Pharmaceuticals (all unrelated to the present work). Dr Honigberg has received consulting fees from Comanche Biopharma; has served on an advisory board for Miga Health; has worked as site principal investigator for Novartis; and has received research support from Genentech. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


References
Publication types
MeSH terms
Grants and funding
- U10 HL120006/HL/NHLBI NIH HHS/United States
- U10 HL119991/HL/NHLBI NIH HHS/United States
- U10 HL120018/HL/NHLBI NIH HHS/United States
- U10 HD063037/HD/NICHD NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- U01 HL145358/HL/NHLBI NIH HHS/United States
- U10 HL119993/HL/NHLBI NIH HHS/United States
- U10 HL119992/HL/NHLBI NIH HHS/United States
- U10 HD063053/HD/NICHD NIH HHS/United States
- U10 HL120034/HL/NHLBI NIH HHS/United States
- U10 HL120019/HL/NHLBI NIH HHS/United States
- U10 HD063036/HD/NICHD NIH HHS/United States
- U10 HL119989/HL/NHLBI NIH HHS/United States
- K08 HL166687/HL/NHLBI NIH HHS/United States
- U10 HD063046/HD/NICHD NIH HHS/United States
- U10 HD063072/HD/NICHD NIH HHS/United States
- U10 HD063048/HD/NICHD NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- U10 HD063047/HD/NICHD NIH HHS/United States
- U10 HL119990/HL/NHLBI NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- U10 HD063041/HD/NICHD NIH HHS/United States
- U10 HD063020/HD/NICHD NIH HHS/United States
- UL1 TR000153/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

