Changes of Clinical Practice Patterns of Allergen Immunotherapy in Korea
- PMID: 40204510
- PMCID: PMC11982636
- DOI: 10.4168/aair.2025.17.2.271
Changes of Clinical Practice Patterns of Allergen Immunotherapy in Korea
Abstract
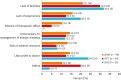
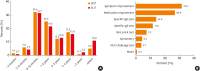
This study aimed to identify recent changes of AIT treatment behaviors in real-world clinical practice using a questionnaire survey in Korea. The questionnaire on AIT prescriptions and practical experiences was distributed to all members of the Korean Academy of Asthma Allergy and Clinical Immunology in June 2022. The responses were analyzed and compared with the results from 2009 and 2017. In total, 115 responses (10.1%) were collected; 58 (50.4%) from internal medicine, 34 (29.6%) from pediatricians, and 21 (18.3%) from otolaryngologists. The prescription rate for subcutaneous immunotherapy (SCIT) was 53.8%, showing a decrease from those in 2009 and 2017; however, that for sublingual immunotherapy (SLIT) increased steadily, reaching 17.9% in 2009, 40.3% in 2017, and 46.2% in 2022. The prescription rates for asthma and atopic dermatitis increased by 4.6% and 7.9%, respectively. The most frequently prescribed allergens for SCIT in 2022 were house dust mites (32.9%), pollen (30.6%), and animal dander (28.2%), with the rate for animal dander showing a significant increase from 10.3% in 2009. Most physicians (93%) used mixed allergens for SCIT, with 42.8% using a combination of 5 or more allergens. Fifty-eight (67.4%) respondents reported cases of anaphylaxis during SCIT and 36.2% reported systemic adverse reactions during SLIT. In conclusion, SLIT prescriptions, AIT for asthma and atopic dermatitis, and AIT with animal dander increased significantly from 2009 to 2022. Serial surveys of AIT practices are helpful in identifying the changes of real-world clinical practice of AIT.
Keywords: Allergens; allergic rhinitis; asthma; atopic dermatitis; immunotherapy; surveys and questionnaires.
Copyright © 2025 The Korean Academy of Asthma, Allergy and Clinical Immunology • The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures
References
-
- Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. - PubMed
-
- Durham SR, Emminger W, Kapp A, de Monchy JG, Rak S, Scadding GK, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–725.e5. - PubMed
-
- Niggemann B, Jacobsen L, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61:855–859. - PubMed
-
- Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Macchia L, Di Lorenzo G. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691–704. - PubMed

