Measurement of Twitch Dynamics in Response to Exercise Induced Changes in Mitochondrial Disease Using Motor Unit Magnetic Resonance Imaging (MUMRI): A Proof-of-Concept Study
- PMID: 40204627
- PMCID: PMC11981886
- DOI: 10.1002/nbm.70021
Measurement of Twitch Dynamics in Response to Exercise Induced Changes in Mitochondrial Disease Using Motor Unit Magnetic Resonance Imaging (MUMRI): A Proof-of-Concept Study
Abstract
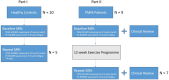
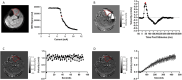
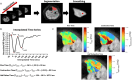
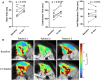
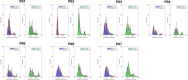
Muscle twitch dynamics and fatigability change in response to muscle disease. In this study, we developed an imaging paradigm to measure muscle twitch dynamics, and the response of the muscle to voluntary fatiguing contractions. We used a novel imaging technique called motor unit magnetic resonance imaging (MUMRI). MUMRI allows visualisation of muscle and motor unit activity by combining in-scanner electrical stimulation with dynamic pulsed gradient spin echo (twitch dynamics, PGSE-MUMRI) and phase contrast (fatigue, PC-MUMRI) imaging. In Part I of this study, we scanned 10 healthy controls, we measured the muscle rise (Trise), contraction (Tcontract) and half-relaxation time (Thalf-relax) of the tibialis anterior (TA) muscle on a voxel-wise basis using PGSE-MUMRI. Five controls were scanned twice to assess reproducibility; PGSE-MUMRI demonstrated reproducible results, with low variation between scans 3.4% for Trise, 6.4% for Tcontract and 7.1% for Thalf-relax. We then developed a PC-MUMRI paradigm to measure the recovery of the TA in response to a fatiguing voluntary exercise. In Part II of the study, we applied these two novel imaging paradigms in a cohort study of nine patients with single large-scale mtDNA deletion primary mitochondrial myopathy (PMM). Patients underwent a 12-week resistance exercise programme and baseline, and follow-up MRI was performed. PGSE-MUMRI detected a significantly longer muscle contraction time between baseline and follow-up in PMM patients 108.7 ± 7.9 vs. post-119.3 ± 10.4 ms; p = 0.018. There was no statistical difference in the recovery half maximum measured using PC-MUMRI in PMM patients between baseline and follow-up 254 ± 109 vs. 137 ± 41 s; p = 0.074. In conclusion, PGSE-MUMRI has detected differences in muscle twitch dynamics between controls and PMM following an exercise programme, and we can visualise differences in twitch dynamics subregions of muscle using this technique. The PC-MUMRI technique has shown promise as a novel measure of muscle fatigue.
Keywords: exercise; fatigue; mitochondrial disease; motor unit; twitch dynamics.
© 2025 The Author(s). NMR in Biomedicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Taivassalo T., De Stefano N., Argov Z., et al., “Effects of Aerobic Training in Patients With Mitochondrial Myopathies,” Neurology 50 (1998): 1055–1060. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

