Combined Treatment With Cognitive Behavioral Therapy for Insomnia and Acceptance and Commitment Therapy Enhances Objective and Subjective Reports of Sleep in Patients With Advanced Cancer
- PMID: 40204663
- PMCID: PMC11981972
- DOI: 10.1002/pon.70141
Combined Treatment With Cognitive Behavioral Therapy for Insomnia and Acceptance and Commitment Therapy Enhances Objective and Subjective Reports of Sleep in Patients With Advanced Cancer
Abstract
Background: Sleep difficulties are common for people with advanced cancer and are associated with poorer mood, lower quality of life, and reduced survival. For these patients, insomnia severity ratings are tied to nighttime awakenings, but little is known about the reasons for awakenings.
Aims: This study reports actigraphy sleep outcomes, longitudinal self-reported insomnia severity, and circadian rhythm disruptions from a randomized pilot study comparing a multi-symptom intervention with a wait-list control group for people with advanced cancer.
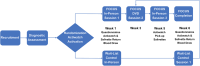
Methods: Twenty-eight people with advanced cancer completed a brief intervention, Finding Our Center Under Stress (FOCUS), designed to enhance sleep and alleviate worry, depression, and fatigue. Participants completed questionnaires and wore an Actiwatch for 7 consecutive 24-h periods pre- and post-intervention.
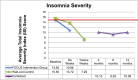
Results: There were no significant group × time actigraphy effects. However, sensitivity analyses with the full intervention sample including the wait-list control arm demonstrated significant effects on actigraphy sleep efficiency, minutes awake after sleep onset (WASO), number of awakenings, naps, and activity at rest. Insomnia severity ratings on the Insomnia Severity Index were maintained longitudinally with 61% meeting the cut-off for insomnia at baseline compared to 18% at 1 year. Participants demonstrated reductions in key reasons for awakenings.
Conclusions: Multi-symptom interventions may be necessary for sustained insomnia improvements for people with advanced cancer. The FOCUS intervention is one of the first to demonstrate improvements on self-reported and actigraphic measures of sleep in addition to other symptoms (i.e., worry, uncertainty, depression, fatigue interference, distress) for this population. Future effectiveness studies are warranted given results of this pilot trial.
Trial registration: Cognitive-behavioral intervention for worry, uncertainty, and insomnia for cancer survivors (NCT01929720).
Keywords: acceptance and commitment therapy; cancer; cognitive behavioral therapy; depression; fatigue; oncology; sleep; uncertainty; worry.
© 2025 The Author(s). Psycho‐Oncology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
- UL1 TR001070/TR/NCATS NIH HHS/United States
- IRG-67-003-47/American Cancer Society Grant
- Tzagournis Medical Research Endowment Fund
- CCP194/Ohio State Comprehensive Cancer Center Cancer Control Grant
- UL1TR001070/Ohio State Clinical Research Center via the National Center for Advancing Translational Sciences Grant
LinkOut - more resources
Full Text Sources
Medical

