Monocyte-macrophage membrane expression of IL-1R2 is a severity biomarker in sepsis
- PMID: 40204720
- PMCID: PMC11982311
- DOI: 10.1038/s41419-025-07597-x
Monocyte-macrophage membrane expression of IL-1R2 is a severity biomarker in sepsis
Abstract
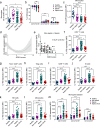
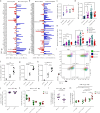
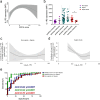
Interleukin-1 (IL-1)/IL-1 receptor family consists of activators and inhibitors which play a key role in inflammation, emergency myelopoiesis, and myeloid cell activation. The latter includes the IL-1R2 decoy receptor. To investigate the expression and significance of IL-1R2 in sepsis, we conducted high-dimensional flow cytometry of circulating cells from patients stratified according to the Sequential Sepsis-Related Organ Failure Assessment (SOFA) score. Here we report that the IL-1 decoy receptor is selectively upregulated on the plasma membrane of leukocytes and, in particular, monocytes from septic patients, and downregulated in septic shock. Flow cytometry combined with transcriptomic analysis of publicly available datasets indicated that IL-1R2 is associated with the differentiation of monocytes to a population of circulating monocytic cells with macrophage features (Mono/Mφ). In vitro stimulation of monocytes from healthy donors with Colony Stimulating Factors (CSFs), in particular GM-CSF and Lipopolysaccharides (LPS), induced IL-1R2+ Mono/Mφ, which recapitulated the characteristics of sepsis-associated monocytic cells, including low expression of HLA-DR, high levels of macrophage markers such as MS4A4A and CD63, immune checkpoints, immunosuppressive molecules and selected scavenger receptors. Membrane-associated IL-1R2 and MS4A4A correlated with immunological markers, cytokine storm, and clinical parameters (e.g., SOFA score, creatinine, survival), reflecting the infection severity in hospitalized patients.Thus, in sepsis IL-1R2 is expressed in a subset of circulating monocytes co-expressing mature macrophage and immune dysfunction features with clinical significance.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval and Consent for publication: This study complied with the provisions of the Declaration of Helsinki and was approved by the Institutional Review Board of Humanitas Research Hospital (Approval n° 820/18). Patients were enrolled only after the signature of a written informed consent. In this case, the patient was unable to provide consent, this was obtained from their relatives. Confidentiality of patient data was preserved, and no patient identifiers were used in the dataset.
Figures

References
-
- Rubio I, Osuchowski MF, Shankar-Hari M, Skirecki T, Winkler MS, Lachmann G, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis. 2019;19:e422–e36. - PubMed
-
- van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–20. - PubMed
-
- Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75–87. - PubMed
-
- van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54:2450–64. - PubMed
MeSH terms
Substances
Grants and funding
- Project no. PE00000007, INF-ACT/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
- PNC-E3-2022-23683266-CUP E23C22000960001/Ministero della Salute (Ministry of Health, Italy)
- project code 29771/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- grant IG-2019 N. 23465/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- H2020-MSCA-ITN-2015, Grant agreement Number: 676129/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

