An unsupervised map of excitatory neuron dendritic morphology in the mouse visual cortex
- PMID: 40204760
- PMCID: PMC11982532
- DOI: 10.1038/s41467-025-58763-w
An unsupervised map of excitatory neuron dendritic morphology in the mouse visual cortex
Abstract
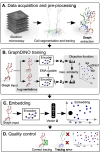
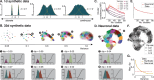
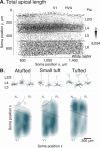
Neurons in the neocortex exhibit astonishing morphological diversity, which is critical for properly wiring neural circuits and giving neurons their functional properties. However, the organizational principles underlying this morphological diversity remain an open question. Here, we took a data-driven approach using graph-based machine learning methods to obtain a low-dimensional morphological "bar code" describing more than 30,000 excitatory neurons in mouse visual areas V1, AL, and RL that were reconstructed from the millimeter scale MICrONS serial-section electron microscopy volume. Contrary to previous classifications into discrete morphological types (m-types), our data-driven approach suggests that the morphological landscape of cortical excitatory neurons is better described as a continuum, with a few notable exceptions in layers 5 and 6. Dendritic morphologies in layers 2-3 exhibited a trend towards a decreasing width of the dendritic arbor and a smaller tuft with increasing cortical depth. Inter-area differences were most evident in layer 4, where V1 contained more atufted neurons than higher visual areas. Moreover, we discovered neurons in V1 on the border to layer 5, which avoided deeper layers with their dendrites. In summary, we suggest that excitatory neurons' morphological diversity is better understood by considering axes of variation than using distinct m-types.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.S.T is a cofounder of Vathes Inc. and UploadAI LLC, companies in which he has financial interests. J.R. is co-founder of Vathes Inc. and UploadAI LLC, companies in which he has financial interests. A.S.E. is a cofounder of Maddox AI GmbH, in which he has financial interests. TM and HSS disclose financial interests in Zetta AI LLC. The remaining authors declare no competing interests.
Figures




References
-
- Ramón y Cajal, S. Histologie du système nerveux de l’homme et des vertébrés. (Ed. Maloine, A.) https://gallica.bnf.fr/ark:/12148/bpt6k6213192g (Paris, 1911).
-
- O’Leary, J. L. Structure of the area striata of the cat. J. Comp. Neurol.75, 131–164 (1941).
MeSH terms
Grants and funding
- 390727645/Deutsche Forschungsgemeinschaft (German Research Foundation)
- D16PC00003/ODNI | Intelligence Advanced Research Projects Activity (IARPA)
- RF1 MH130416/MH/NIMH NIH HHS/United States
- T32-EY-002520-37/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- P30 EY002520/EY/NEI NIH HHS/United States
- 101039115/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- R01 MH109556/MH/NIMH NIH HHS/United States
- D16PC00005/ODNI | Intelligence Advanced Research Projects Activity (IARPA)
- U19 MH114830/MH/NIMH NIH HHS/United States
- R01 EY026927/EY/NEI NIH HHS/United States
- U19MH114830/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- D16PC00004/ODNI | Intelligence Advanced Research Projects Activity (IARPA)
- 101041669/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
LinkOut - more resources
Full Text Sources

