A paper-based loop-mediated isothermal amplification assay for highly pathogenic avian influenza
- PMID: 40204842
- PMCID: PMC11982278
- DOI: 10.1038/s41598-025-95452-6
A paper-based loop-mediated isothermal amplification assay for highly pathogenic avian influenza
Abstract
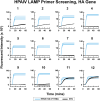
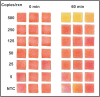
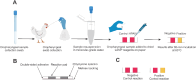
Avian influenza outbreaks have had significant economic and public health consequences worldwide. Therefore, prompt, reliable, and cost-effective diagnostic devices are crucial for scrutinizing and confining highly pathogenic avian influenza viruses (HPAIVs). Our study introduced and evaluated a novel paper-based loop-mediated isothermal amplification (LAMP) test for diagnosing the H5 subtype of the avian influenza virus (AIV). We meticulously designed and screened LAMP primers targeting the H5-haemagglutinin (H5-HA) gene of AIV and fine-tuned the paper-based detection assay for best performance. The paper-based LAMP assay demonstrated a detection limit of 500 copies per reaction (25 copies/µl). Additionally, the assay exhibited no cross-reactivity with common bovine and avian pathogens, confirming its specificity. Spiking experiments revealed that the assay could accurately detect 1000 copies of synthetic HPAIV RNA (per reaction) when spiked into oropharyngeal swab samples, achieving 100% analytical sensitivity, specificity, and accuracy. This inexpensive, user-friendly point-of-need diagnostic tool holds great promise, especially in resource-limited settings. It only requires a water bath for incubation and enables visual detection of results without special equipment. Overall, the paper-based LAMP assay provides a promising method for rapidly and reliably detecting the H5 subtype of AIV, contributing to improved surveillance and early intervention strategies.
Keywords: Diagnostics; Influenza A viruses; Loop-mediated isothermal amplification; Microfluidic paper-based analytical devices; Point-of-care; µPADs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: M.S.V. has interests in Krishi Inc., which is a startup company developing molecular assays. Krishi Inc. did not fund this work. Remaining authors do not have a competing interest.
Figures


Update of
-
A Paper-based Loop-Mediated Isothermal Amplification (LAMP) Assay for Highly Pathogenic Avian Influenza.bioRxiv [Preprint]. 2024 Aug 12:2024.08.12.607641. doi: 10.1101/2024.08.12.607641. bioRxiv. 2024. Update in: Sci Rep. 2025 Apr 9;15(1):12110. doi: 10.1038/s41598-025-95452-6. PMID: 39211221 Free PMC article. Updated. Preprint.
Similar articles
-
A Paper-based Loop-Mediated Isothermal Amplification (LAMP) Assay for Highly Pathogenic Avian Influenza.bioRxiv [Preprint]. 2024 Aug 12:2024.08.12.607641. doi: 10.1101/2024.08.12.607641. bioRxiv. 2024. Update in: Sci Rep. 2025 Apr 9;15(1):12110. doi: 10.1038/s41598-025-95452-6. PMID: 39211221 Free PMC article. Updated. Preprint.
-
Visual detection of H3 subtype avian influenza viruses by reverse transcription loop-mediated isothermal amplification assay.Virol J. 2011 Jul 5;8:337. doi: 10.1186/1743-422X-8-337. Virol J. 2011. PMID: 21729297 Free PMC article.
-
Subpopulation Primers Essential for Exhaustive Detection of Diverse Hemagglutinin Genes of H5 Subtype Avian Influenza Viruses by Loop-Mediated Isothermal Amplification Method.J Clin Microbiol. 2018 Aug 27;56(9):e00985-18. doi: 10.1128/JCM.00985-18. Print 2018 Sep. J Clin Microbiol. 2018. PMID: 30021821 Free PMC article.
-
Advances in the molecular based techniques for the diagnosis and characterization of avian influenza virus infections.Transbound Emerg Dis. 2008 Oct;55(8):329-38. doi: 10.1111/j.1865-1682.2008.01047.x. Transbound Emerg Dis. 2008. PMID: 18786072 Review.
-
The genetics of highly pathogenic avian influenza viruses of subtype H5 in Germany, 2006-2020.Transbound Emerg Dis. 2021 May;68(3):1136-1150. doi: 10.1111/tbed.13843. Epub 2020 Sep 29. Transbound Emerg Dis. 2021. PMID: 32964686 Review.
Cited by
-
Development of a Paper-Based Microfluidic Chip for Point-of-Care Detection of PEDV.Vet Sci. 2025 Apr 30;12(5):427. doi: 10.3390/vetsci12050427. Vet Sci. 2025. PMID: 40431520 Free PMC article.
-
The emergence of highly pathogenic avian influenza H5N1 in dairy cattle: implications for public health, animal health, and pandemic preparedness.Eur J Clin Microbiol Infect Dis. 2025 Aug;44(8):1817-1833. doi: 10.1007/s10096-025-05147-z. Epub 2025 May 14. Eur J Clin Microbiol Infect Dis. 2025. PMID: 40369349 Free PMC article. Review.
References
-
- Badara, O. Wildlife under threat as avian influenza reaches Antarctica. WOAH - World Organisation for Animal Health. https://www.woah.org/en/wildlife-under-threat-as-avian-influenza-reaches... (2024).
-
- USDA, A. USDA APHIS|USDA Confirms Highly Pathogenic Avian Influenza in a Commercial Turkey Flock in Dubois County, Indiana. (2023).
-
- APHIS. Highly Pathogenic Avian Influenza (HPAI) Detections in Livestock | Animal and Plant Health Inspection Service. https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influen... (2024).
-
- AVMA. Avian influenza virus type A (H5N1) in U.S. dairy cattle|American Veterinary Medical Association. https://www.avma.org/resources-tools/animal-health-and-welfare/animal-he... (2024).
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

