Role of phospholipase Cη1 in lateral habenula astrocytes in depressive-like behavior in mice
- PMID: 40204881
- PMCID: PMC12046024
- DOI: 10.1038/s12276-025-01432-1
Role of phospholipase Cη1 in lateral habenula astrocytes in depressive-like behavior in mice
Abstract
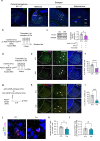
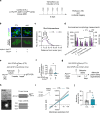
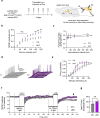
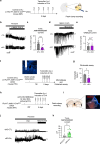
Phospholipase C (PLC) enzymes play crucial roles in intracellular calcium-signaling transduction. Several brain PLC subtypes have been extensively studied, implicating them in psychiatric disorders such as depression, epilepsy and schizophrenia. However, the role of the recently identified PLCη remains largely unknown. We found that PLCη1 is prominently expressed in lateral habenula (LHb) astrocytes. Here, to investigate its physiological role, we generated astrocyte-specific PLCη1 conditional knockout (cKO) mice (Plch1f/f; Aldh1l1-CreERT2). In these cKO mice, we observed a reduction in cellular morphological complexity metrics, such as total process length, as well as a decrease in the passive membrane conductance of LHb astrocytes. Additionally, neuronal function was impacted by the cKO, as the synaptic efficacy and firing rates of LHb neurons increased, while extrasynaptic long-term depression was impaired. Both tonic α-amino-3-hydroxy-5-methyl-4-isoxazolepdlropionic acid receptor/N-methyl-D-aspartate receptor (AMPAR/NMDAR) currents and extracellular glutamate levels were reduced. Interestingly, chemogenetic activation of astrocytes restored the reduced tonic AMPAR/NMDAR currents in cKO mice. Furthermore, LHb astrocyte-specific deletion of PLCη1 via AAV-GFAP-Cre injection induced depressive-like behaviors in mice, which were reversed by chemogenetic activation of LHb astrocytes. Finally, we found that restraint stress exposure decreased Plch1 mRNA expression in the LHb. These findings suggest that PLCη1 could be a potential therapeutic target for depression and highlight the critical role of astrocytes in the etiology of neuropsychiatric disorders.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors report no competing interests.
Figures

Similar articles
-
Neuronal TCF7L2 in Lateral Habenula Is Involved in Stress-Induced Depression.Int J Mol Sci. 2024 Nov 19;25(22):12404. doi: 10.3390/ijms252212404. Int J Mol Sci. 2024. PMID: 39596468 Free PMC article.
-
NMDA receptor-dependent long-term depression in the lateral habenula: implications in physiology and depression.Sci Rep. 2020 Oct 21;10(1):17921. doi: 10.1038/s41598-020-74496-w. Sci Rep. 2020. PMID: 33087756 Free PMC article.
-
Neuron-astrocyte coupling in lateral habenula mediates depressive-like behaviors.Cell. 2025 Jun 12;188(12):3291-3309.e24. doi: 10.1016/j.cell.2025.04.010. Epub 2025 Apr 24. Cell. 2025. PMID: 40280131
-
Tonic NMDA Receptor Currents in the Brain: Regulation and Cognitive Functions.Biol Psychiatry. 2024 Aug 1;96(3):164-175. doi: 10.1016/j.biopsych.2024.03.009. Epub 2024 Mar 13. Biol Psychiatry. 2024. PMID: 38490367 Review.
-
Overcoming Depression by Inhibition of Neural Burst Firing.Neuron. 2018 Jun 6;98(5):878-879. doi: 10.1016/j.neuron.2018.05.032. Neuron. 2018. PMID: 29879390 Review.
Cited by
-
Direct and Indirect Downstream Pathways That Regulate Repulsive Guidance Effects of FGF3 on Developing Thalamocortical Axons.Int J Mol Sci. 2025 Jul 30;26(15):7361. doi: 10.3390/ijms26157361. Int J Mol Sci. 2025. PMID: 40806490 Free PMC article.
References
-
- Rhee, S. G. & Choi, K. D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J. Biol. Chem.267, 12393–12396 (1992). - PubMed
-
- Rebecchi, M. J. & Pentyala, S. N. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev.80, 1291–1335 (2000). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

