Site-directed multivalent conjugation of antibodies to ubiquitinated payloads
- PMID: 40204992
- PMCID: PMC12270900
- DOI: 10.1038/s41551-024-01342-z
Site-directed multivalent conjugation of antibodies to ubiquitinated payloads
Abstract
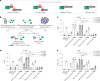
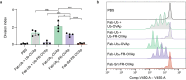
Antibody conjugates are the foundation of a wide range of diagnostic and therapeutic applications. Although many antibody-conjugation techniques are robust and efficient, obtaining homogeneous multimeric conjugation products remains challenging. Here we report a modular and versatile technique for the site-directed multivalent conjugation of antibodies via the small-protein ubiquitin. Specifically, multiple ubiquitin fusions with antibodies, antibody fragments, nanobodies, peptides or small molecules such as fluorescent dyes can be conjugated to antibodies and nanobodies within 30 min. The technique, which we named 'ubi-tagging', allowed us to efficiently generate a bispecific T-cell engager as well as nanobodies conjugated to dendritic-cell-targeted antigens that led to potent T-cell responses. Using both recombinant ubi-tagged proteins and synthetic ubiquitin derivatives allows for the iterative, site-directed and multivalent conjugation of antibodies and nanobodies to a plethora of molecular moieties.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- Yamada, K. & Ito, Y. Recent chemical approaches for site-specific conjugation of native antibodies: technologies toward next-generation antibody–drug conjugates. Chembiochem20, 2729–2737 (2019). - PubMed
-
- Shen, B.-Q. et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody–drug conjugates. Nat. Biotechnol.30, 184–189 (2012). - PubMed
-
- Strop, P. et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem. Biol.20, 161–167 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

