Stress dynamically modulates neuronal autophagy to gate depression onset
- PMID: 40205038
- PMCID: PMC12058529
- DOI: 10.1038/s41586-025-08807-4
Stress dynamically modulates neuronal autophagy to gate depression onset
Erratum in
-
Publisher Correction: Stress dynamically modulates neuronal autophagy to gate depression onset.Nature. 2025 May;641(8065):E14. doi: 10.1038/s41586-025-09086-9. Nature. 2025. PMID: 40335704 Free PMC article. No abstract available.
-
Author Correction: Stress dynamically modulates neuronal autophagy to gate depression onset.Nature. 2025 Aug;644(8075):E12. doi: 10.1038/s41586-025-09404-1. Nature. 2025. PMID: 40696184 Free PMC article. No abstract available.
Abstract
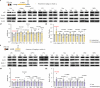
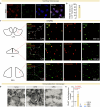
Chronic stress remodels brain homeostasis, in which persistent change leads to depressive disorders1. As a key modulator of brain homeostasis2, it remains elusive whether and how brain autophagy is engaged in stress dynamics. Here we discover that acute stress activates, whereas chronic stress suppresses, autophagy mainly in the lateral habenula (LHb). Systemic administration of distinct antidepressant drugs similarly restores autophagy function in the LHb, suggesting LHb autophagy as a common antidepressant target. Genetic ablation of LHb neuronal autophagy promotes stress susceptibility, whereas enhancing LHb autophagy exerts rapid antidepressant-like effects. LHb autophagy controls neuronal excitability, synaptic transmission and plasticity by means of on-demand degradation of glutamate receptors. Collectively, this study shows a causal role of LHb autophagy in maintaining emotional homeostasis against stress. Disrupted LHb autophagy is implicated in the maladaptation to chronic stress, and its reversal by autophagy enhancers provides a new antidepressant strategy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


















References
-
- Herrman, H. et al. Time for united action on depression: a Lancet–World Psychiatric Association Commission. Lancet399, 957–1022 (2022). - PubMed
-
- Soykan, T., Haucke, V. & Kuijpers, M. Mechanism of synaptic protein turnover and its regulation by neuronal activity. Curr. Opin. Neurobiol.69, 76–83 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

