Heterogeneity of predictive biomarker expression in gastric and esophago-gastric junction carcinoma with peritoneal dissemination
- PMID: 40205072
- PMCID: PMC12174275
- DOI: 10.1007/s10120-025-01609-7
Heterogeneity of predictive biomarker expression in gastric and esophago-gastric junction carcinoma with peritoneal dissemination
Abstract
Background: Temporal and spatial molecular heterogeneity contributes to resistance to targeted and immune therapies in gastric and esophagogastric junction carcinoma (G/EGJ). This study evaluates differences in biomarker expression between primary G/EGJ and paired peritoneal metastases (PM).
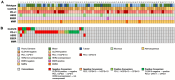
Methods: We analyzed 74 cases of primary G/EGJ and paired PM using immunohistochemistry for HER2, PD-L1, Claudin18 (CLDN18), DNA mismatch repair (MMR) proteins, p53, E-cadherin, and in situ hybridization for EBER. Biomarker concordance between primary and metastatic tumors was assessed.
Results: Primary G/EGJ were predominantly poorly cohesive (45.9%) or mixed-type (37.8%). Regarding predictive biomarkers, low rates of HER2 overexpression (5.4%), MMR deficiency (4.1%), and EBER positivity (1.4%) were observed, while PD-L1 CPS ≥ 1 occurred in 79.7% of cases and CLDN18 positivity was observed in 31.1% of cases. Concordance was perfect for MMR and EBER, while PD-L1 showed the highest discordance (32.4%). HER2 had a low discordance rate (2.7%). CLDN18 exhibited good concordance (86.5%) and showed consistent positivity in PD-L1- and HER2-negative primary tumors (28.6%).
Conclusion: G/EGJ with PM show distinct molecular features and spatial heterogeneity, with MMR, EBER, and HER2 demonstrating strong concordance, while PD-L1 showed greater variability. As for novel biomarkers, CLDN18.2 shows substantial concordance between primary G/EGJ and PM and could be a promising target in HER2/PD-L1-negative G/EGJ with PM.
Keywords: Biomarkers; Gastroesophageal cancer; Heterogeneity; Targeted therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article. Ethical approval and consent to participate: All clinical specimens used in this study were approved by our Institutional Review Board, and was included in the observational retrospective study “GAS-ALL-IN—GAStric cancers a retrospective analysis of ALL major prognostic and predictive determINants”. The study was performed according to the clinical standards of the 1975 and 1983 Declaration of Helsinki. Consent for publication: Not applicable.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

