Plasma phospho-tau217 for Alzheimer's disease diagnosis in primary and secondary care using a fully automated platform
- PMID: 40205199
- PMCID: PMC12176611
- DOI: 10.1038/s41591-025-03622-w
Plasma phospho-tau217 for Alzheimer's disease diagnosis in primary and secondary care using a fully automated platform
Abstract
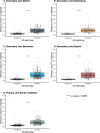
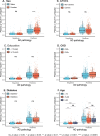
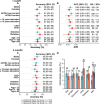
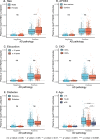
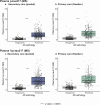
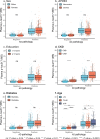
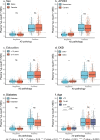
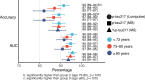
Global implementation of blood tests for Alzheimer's disease (AD) would be facilitated by easily scalable, cost-effective and accurate tests. In the present study, we evaluated plasma phospho-tau217 (p-tau217) using predefined biomarker cutoffs. The study included 1,767 participants with cognitive symptoms from 4 independent secondary care cohorts in Malmö (Sweden, n = 337), Gothenburg (Sweden, n = 165), Barcelona (Spain, n = 487) and Brescia (Italy, n = 230), and a primary care cohort in Sweden (n = 548). Plasma p-tau217 was primarily measured using the fully automated, commercially available, Lumipulse immunoassay. The primary outcome was AD pathology defined as abnormal cerebrospinal fluid Aβ42:p-tau181. Plasma p-tau217 detected AD pathology with areas under the receiver operating characteristic curves of 0.93-0.96. In secondary care, the accuracies were 89-91%, the positive predictive values 89-95% and the negative predictive values 77-90%. In primary care, the accuracy was 85%, the positive predictive values 82% and the negative predictive values 88%. Accuracy was lower in participants aged ≥80 years (83%), but was unaffected by chronic kidney disease, diabetes, sex, APOE genotype or cognitive stage. Using a two-cutoff approach, accuracies increased to 92-94% in secondary and primary care, excluding 12-17% with intermediate results. Using the plasma p-tau217:Aβ42 ratio did not improve accuracy but reduced intermediate test results (≤10%). Compared with a high-performing mass-spectrometry-based assay for percentage p-tau217, accuracies were comparable in secondary care. However, percentage p-tau217 had higher accuracy in primary care and was unaffected by age. In conclusion, this fully automated p-tau217 test demonstrates high accuracy for identifying AD pathology. A two-cutoff approach might be necessary to optimize performance across diverse settings and subpopulations.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.P. acquired research support (for the institution) from Avid and ADDF through ki elements. In the past 2 years, he received consultancy or speaker fees from BioArtic, Biogen, Eisai, Eli Lilly, Novo Nordisk and Roche. N.M.-C. received consultancy/speaker from Biogen, Owkin and Merck. R.S. received speaker fees from Roche and Triolab. H.Z. served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). R.O. received research funding or support from the ERC, ZonMw, NWO, National Institutes of Health, Alzheimer Association, Alzheimer Nederland, Stichting Dioraphte, Cure Alzheimer’s fund, Health Holland, ERA PerMed, Alzheimerfonden, Hjarnfonden, Avid Radiopharmaceuticals, Janssen Research & Development, Roche, Quanterix and Optina Diagnostics, has given lectures in symposia sponsored by GE Healthcare and is an advisory board member for Asceneuron and a steering committee member for Bristol Myers Squibb. All the aforementioned have been paid to the institutions. C.T. is supported by the Ministry of Health PRIN 2021 RePlast. A.P.‐P. served on advisory boards for Schwabe Farma Iberica. S.K. served on scientific advisory boards, and as a speaker and/or consultant for Roche, Eli Lilly, Geras Solutions, Optoceutics, Biogen, Eisai, Merry Life, Triolab, Novo Nordisk and BioArctic. A. Pilotto received travel grants from Abbvie, Bial, Lundbeck, Roche and Zambon pharmaceuticals, and personal compensation as a consultant or fees for lectures from Abbvie, Biogen and Lundbeck. A. Padovani received travel grants from Biougen, Lundbeck, Novonordisk, Roche pharmaceuticals, and personal compensation as a consultant or fees for lectures from Biogen, Lundbeck, Roche, Nutricia and General Healthcare (GE). K.B. served as a consultant and was on advisory boards for Acumen, ALZpath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics and Siemens Healthineers, served at data monitoring committees for Julius Clinical and Novartis, has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics, and is a co-founder of BBS, which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. N.J.A. received consultancy or speaker fees from BioArtic, Biogen, Lilly, Quanterix and Alamar Biosciences. R.S. received a speaker’s fee from Roche. M.S.-C. received, in the past 36 months, consultancy or speaker’s fees (paid to the institution) from Almirall, Eli Lilly, Novo Nordisk and Roche Diagnostics. He received consultancy fees or served on advisory boards (paid to the institution) of Eli Lilly, Grifols, Novo Nordisk and Roche Diagnostics. He was granted a project and is a site investigator of a clinical trial (funded to the institution) by Roche Diagnostics. In-kind support for research (to the institution) was received from ADx Neurosciences, Alamar Biosciences, ALZpath, Avid Radiopharmaceuticals, Eli Lilly, Fujirebio, Janssen Research & Development, Meso Scale Discovery and Roche Diagnostics. M.S.-C. did not receive any personal compensation from these organizations or any other for-profit organization. O.H. is an employee of Eli Lilly and Lund University and previously acquired research support (for Lund University) from AVID Radiopharmaceuticals, Biogen, C2N Diagnostics, Eli Lilly, Eisai, Fujirebio, GE Healthcare and Roche. In the past 2 years, he received consultancy or speaker’s fees from ALZpath, BioArctic, Biogen, Bristol Meyer Squibb, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. All C2N coauthors are salaried employees or consultants with cash and/or equity compensation from C2N Diagnostics. C2N Diagnostics performed the MS analyses blinded to any biomarker or clinical data and had no role in the statistical analysis or results. The other authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
- ZEN24-1069572/ALZ/Alzheimer's Association/United States
- 2018-02052/Vetenskapsrådet (Swedish Research Council)
- R01AG083740/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- FO2024-0284/Hjärnfonden (Swedish Brain Foundation)
- R01 AG083740/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

