Connectomics of predicted Sst transcriptomic types in mouse visual cortex
- PMID: 40205210
- PMCID: PMC11981948
- DOI: 10.1038/s41586-025-08805-6
Connectomics of predicted Sst transcriptomic types in mouse visual cortex
Abstract
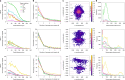
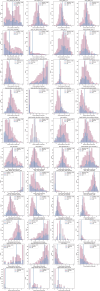
Neural circuit function is shaped both by the cell types that comprise the circuit and the connections between them1. Neural cell types have previously been defined by morphology2,3, electrophysiology4, transcriptomic expression5,6, connectivity7-9 or a combination of such modalities10-12. The Patch-seq technique enables the characterization of morphology, electrophysiology and transcriptomic properties from individual cells13-15. These properties were integrated to define 28 inhibitory, morpho-electric-transcriptomic (MET) cell types in mouse visual cortex16, which do not include synaptic connectivity. Conversely, large-scale electron microscopy (EM) enables morphological reconstruction and a near-complete description of a neuron's local synaptic connectivity, but does not include transcriptomic or electrophysiological information. Here, we leveraged morphological information from Patch-seq to predict the transcriptomically defined cell subclass and/or MET-type of inhibitory neurons within a large-scale EM dataset. We further analysed Martinotti cells-a somatostatin (Sst)-positive17 morphological cell type18,19-which were classified successfully into Sst MET-types with distinct axon myelination and synaptic output connectivity patterns. We demonstrate that morphological features can be used to link cell types across experimental modalities, enabling further comparison of connectivity to gene expression and electrophysiology. We observe unique connectivity rules for predicted Sst cell types.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: N.K., K. Lee, T.M. and H.S.S. disclose financial interests in Zetta AI LLC. The remaining authors declare no competing interests.
Figures









References
-
- Zeng, H. & Sanes, J. R. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat. Rev. Neurosci.18, 530–546 (2017). - PubMed
-
- Cajal, S. R. Y., Azoulay, D. L., Swanson, N. & Swanson, L. W. Histology Of The Nervous System: Of Man And Vertebrates (Oxford Univ. Press, 1995).
-
- De Carlos, J. A. & Borrell, J. A historical reflection of the contributions of Cajal and Golgi to the foundations of neuroscience. Brain Res. Rev.55, 8–16 (2007). - PubMed
-
- Zeisel, A. et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science347, 1138–1142 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

