Functional connectomics reveals general wiring rule in mouse visual cortex
- PMID: 40205211
- PMCID: PMC11981947
- DOI: 10.1038/s41586-025-08840-3
Functional connectomics reveals general wiring rule in mouse visual cortex
Abstract
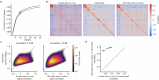
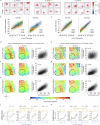
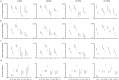
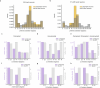
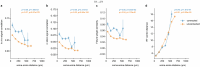
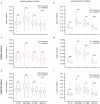
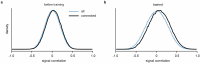
Understanding the relationship between circuit connectivity and function is crucial for uncovering how the brain computes. In mouse primary visual cortex, excitatory neurons with similar response properties are more likely to be synaptically connected1-8; however, broader connectivity rules remain unknown. Here we leverage the millimetre-scale MICrONS dataset to analyse synaptic connectivity and functional properties of neurons across cortical layers and areas. Our results reveal that neurons with similar response properties are preferentially connected within and across layers and areas-including feedback connections-supporting the universality of 'like-to-like' connectivity across the visual hierarchy. Using a validated digital twin model, we separated neuronal tuning into feature (what neurons respond to) and spatial (receptive field location) components. We found that only the feature component predicts fine-scale synaptic connections beyond what could be explained by the proximity of axons and dendrites. We also discovered a higher-order rule whereby postsynaptic neuron cohorts downstream of presynaptic cells show greater functional similarity than predicted by a pairwise like-to-like rule. Recurrent neural networks trained on a simple classification task develop connectivity patterns that mirror both pairwise and higher-order rules, with magnitudes similar to those in MICrONS data. Ablation studies in these recurrent neural networks reveal that disrupting like-to-like connections impairs performance more than disrupting random connections. These findings suggest that these connectivity principles may have a functional role in sensory processing and learning, highlighting shared principles between biological and artificial systems.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.S.T., J.R., E.Y. Walker, and D.Y. are co-founders of DataJoint Inc. in which they have financial interests. The other authors declare no competing interests.
Figures










Update of
-
Functional connectomics reveals general wiring rule in mouse visual cortex.bioRxiv [Preprint]. 2024 Oct 14:2023.03.13.531369. doi: 10.1101/2023.03.13.531369. bioRxiv. 2024. Update in: Nature. 2025 Apr;640(8058):459-469. doi: 10.1038/s41586-025-08840-3. PMID: 36993398 Free PMC article. Updated. Preprint.
References
-
- Wertz, A. et al. Single-cell-initiated monosynaptic tracing reveals layer-specific cortical network modules. Science349, 70–74 (2015). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

