Functional connectomics spanning multiple areas of mouse visual cortex
- PMID: 40205214
- PMCID: PMC11981939
- DOI: 10.1038/s41586-025-08790-w
Functional connectomics spanning multiple areas of mouse visual cortex
Abstract
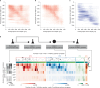
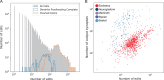
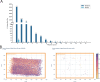
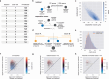
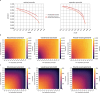
Understanding the brain requires understanding neurons' functional responses to the circuit architecture shaping them. Here we introduce the MICrONS functional connectomics dataset with dense calcium imaging of around 75,000 neurons in primary visual cortex (VISp) and higher visual areas (VISrl, VISal and VISlm) in an awake mouse that is viewing natural and synthetic stimuli. These data are co-registered with an electron microscopy reconstruction containing more than 200,000 cells and 0.5 billion synapses. Proofreading of a subset of neurons yielded reconstructions that include complete dendritic trees as well the local and inter-areal axonal projections that map up to thousands of cell-to-cell connections per neuron. Released as an open-access resource, this dataset includes the tools for data retrieval and analysis1,2. Accompanying studies describe its use for comprehensive characterization of cell types3-6, a synaptic level connectivity diagram of a cortical column4, and uncovering cell-type-specific inhibitory connectivity that can be linked to gene expression data4,7. Functionally, we identify new computational principles of how information is integrated across visual space8, characterize novel types of neuronal invariances9 and bring structure and function together to uncover a general principle for connectivity between excitatory neurons within and across areas10,11.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S. Seung and T. Macrina disclose a competing interest in ZettaAI; J. Reimer and A. S. Tolias disclose a competing interest in Vathes. The other authors declare no competing interests.
Figures






References
-
- Bodor, A. L. et al. The synaptic architecture of layer 5 thick tufted excitatory neurons in the visual cortex of mice. Nat. Neurosci. (in the press).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

