Ciprofol versus propofol for long-term sedation in mechanically ventilated patients with sepsis: a randomized controlled trial
- PMID: 40205333
- PMCID: PMC11983952
- DOI: 10.1186/s12871-025-03042-w
Ciprofol versus propofol for long-term sedation in mechanically ventilated patients with sepsis: a randomized controlled trial
Abstract
Background: Sedatives are often used to facilitate mechanical ventilation in patients with sepsis. Ciprofol is a new promising sedated candidate with a higher binding activity to the gamma-aminobutyric acid-A receptor than propofol. This study aimed to compare the efficacy and safety of ciprofol and propofol for long-term sedation in mechanically ventilated patients with sepsis.
Methods: In this single-center randomized clinical trial, mechanically ventilated adults with sepsis in the intensive care unit (ICU) who anticipated to require long-term sedation ≥ 24 h were randomly assigned to receive intravenous ciprofol or propofol. The target sedation goal was - 3 to 0 according to the Richmond Agitation-Sedation Scale. The primary outcome was weaning time. Secondary outcomes included the percentage of time within the target sedation range, successful sedation (the percentage of time within the target sedation range ≥ 70% without rescue sedation), ICU and in-hospital mortality, length of ICU and hospital stay, hypotension, and bradycardia.
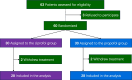
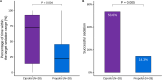
Results: A total of 60 patients were randomized, 4 were excluded because of withdrawing treatment, 28 were assigned to ciprofol group and 28 to propofol group. Weaning time in ciprofol group was shorter than propofol group (median [interquartile range (IQR)], 104.0 [40.8-147.3] hours vs 132.5 [69.8-207.8] hours), but not reached significant difference between groups (P = 0.123). Ciprofol had significantly higher percentage of time within the target sedation range (median [IQR], 72.2% [14.3-92.7%] vs 22.6% [0.0-45.4%]) and successful sedation (53.6% [15/28] vs 14.3% [4/28]) than propofol. No significant differences were observed in ICU mortality, in-hospital mortality, length of ICU stay, length of hospital stay, hypotension, and bradycardia between groups.
Conclusions: Ciprofol is an effective and safe agent among mechanically ventilated patients with sepsis who anticipated to require long-term sedation.
Trial registration number: The trial was registered at the Chinese Clinical Trial Registry (ChiCTR2200066835) on December 19, 2022.
Keywords: Ciprofol; Mechanical ventilation; Propofol; Sedation; Sepsis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This trial has been conducted in accordance with the Declaration of Helsinki. This trial has been approved by the Scientific Research Ethics Committee of the First Affiliated Hospital of Jinan University (KY- 2022–090). The trial was registered at the Chinese Clinical Trial Registry (ChiCTR2200066835) on December 19, 2022. All participants’ legally authorized representatives signed written informed consent before enrollment. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- 21623302/Fundamental Research Funds for the Central Universities
- 2024A04J3706/Funding by Science and Technology Projects in Guangzhou
- 82072232/National Natural Science Foundation of China
- 202201020028/Science and Technology Program of Guangzhou, China
- 2022ZDZX2003/Special Projects in Key Areas of General Colleges and Universities in Guangdong Province
LinkOut - more resources
Full Text Sources
Medical