Clinical impact of concurrent autologous adoptive T cells immunotherapy in active COVID-19 infected cancer patients for chemotherapy
- PMID: 40205403
- PMCID: PMC11983847
- DOI: 10.1186/s13027-025-00654-2
Clinical impact of concurrent autologous adoptive T cells immunotherapy in active COVID-19 infected cancer patients for chemotherapy
Abstract
Background: The concurrent presence of COVID-19 infection in advanced cancer patients has increased the mortality since the compromised immunity was inevitably worsen. The role and clinical impact of autologous adoptive T cell immunotherapy (ACT) designed for anti-cancer treatment were not known in such circumstances. The safety and potential immune reconstitution of concurrent ACT in advanced cancer patients with active COVID-19 infection have yet unknown as well. The effect of infused ACT on the symptom severity manifestation should be summarized.
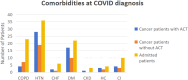
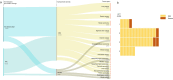
Methods: In this respectively clinical observation study, patients were non-randomized enrolled from the two centers according to the regular therapeutic plans including stage IV cancer patients for scheduled ACT, chemotherapy, cancer patients with symptomatic COVID-19 but without ACT, neither cancer or non-ACT but symptomatic cases of COVID-19 infection. We have incorporated the age-adjusted Charlson comorbidity index (aCCI) for each patient to compare the prognosis of the three groups. All patients were planned for the scheduled standard anti-cancer therapeutic considerations, chemotherapy plus ACT as planned as well as the supportive care.The clinical efficacy and impact of ACT on cancer patients within the 3 months from the peripheral blood apheresis, dendritic cell (DC) and cytokine induced killer T cell (CIK-T ) infusion and subsequent co-existence of COVID-19 infection were recorded as the primary objective. During the same period, the cancer cases without ACT and others were collected to compare the occurrence of both severe and death rate respectively.
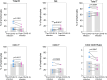
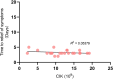
Results: There were 123 patients (35 of ACT, 23 of non-ACT, 65 of non-cancer) with similar aCCI. There were similar cohort-level COVID-19 in-hospital case fatality rates consistent with previously reported data for non-cancer (26.2%, 17/65) and non-ACT cancer (52.2%, 12/23) among those admitted severe cases after the adjustment.There were little overlapped adverse reactions during the ACT therapeutic period even in the presence of active COVID-19 infection. No death case was occurred (0/35) when those exposed to ACT regimen. Cancer patients receiving ACT had a shorter mean time to alleviation of symptoms compared with non-ACT and non-cancer (4.46 versus 16.88 and 17.90 days respectively) as well as the lowered severity incidence of symptoms (P = 0.0010). The infused ACT has not significant impact on peripheral blood count whereas the amount of CD3-CD16+CD56+ NK cells increased (P = 0.0017). The quantity of infused ACT was favorable for augmentation of possibility of severe to mild symptom shift.
Conclusions: These data demonstrate the clinical safety profiles while ACT infusions with active COVID-19 infection.The intervention of ACT for cancer patients could generate the benefit for symptom alleviation with improved recovery time. The concurrent ACT for advanced cancer patients during such infectious pandemic might simultaneously leverage and reduce the risk of immune compromised situation for subsequent chemotherapy complications.
Keywords: Autologous adoptive cellular immunotherapy; COVID-19 infection; Safety..
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Medical Ethics Committee of Fudan University Pudong Medical Center, Shanghai, China. Ethics number: 2021-IIT-021-E02. Consent for publication: Written informed consent to participate in this study was obtained from all patients at the time of admission. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Combination of DC/CIK adoptive T cell immunotherapy with chemotherapy in advanced non-small-cell lung cancer (NSCLC) patients: a prospective patients' preference-based study (PPPS).Clin Transl Oncol. 2019 Jun;21(6):721-728. doi: 10.1007/s12094-018-1968-3. Epub 2018 Oct 29. Clin Transl Oncol. 2019. PMID: 30374838
-
Blood microbiota diversity determines response of advanced colorectal cancer to chemotherapy combined with adoptive T cell immunotherapy.Oncoimmunology. 2021 Sep 27;10(1):1976953. doi: 10.1080/2162402X.2021.1976953. eCollection 2021. Oncoimmunology. 2021. PMID: 34595059 Free PMC article.
-
Clinical effects of autologous dendritic cells combined with cytokine-induced killer cells followed by chemotherapy in treating patients with advanced colorectal cancer: a prospective study.Tumour Biol. 2016 Apr;37(4):4367-72. doi: 10.1007/s13277-015-3957-2. Epub 2015 Oct 24. Tumour Biol. 2016. PMID: 26499782
-
Cytokine-induced killer cells/dendritic cells and cytokine-induced killer cells immunotherapy for the treatment of esophageal cancer: A meta-analysis.Medicine (Baltimore). 2021 Apr 2;100(13):e24519. doi: 10.1097/MD.0000000000024519. Medicine (Baltimore). 2021. PMID: 33787569 Free PMC article.
-
Clinical effect and safety of dendritic cell-cytokine-induced killer cell immunotherapy for pancreatic cancer: a systematic review and meta-analysis.Cytotherapy. 2019 Oct;21(10):1064-1080. doi: 10.1016/j.jcyt.2019.07.006. Epub 2019 Aug 26. Cytotherapy. 2019. PMID: 31462394
References
-
- Khoury E, Nevitt S, Madsen WR, Turtle L, Davies G, Palmieri C. Differences in outcomes and factors associated with mortality among patients with SARS-CoV-2 infection and cancer compared with those without cancer: A systematic review and Meta-analysis. JAMA Netw Open. 2022;5(5):e2210880. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

