Assessing iIndications for herbal medicinal products: a comparative analysis of EMA monographs and database records
- PMID: 40205417
- PMCID: PMC11980217
- DOI: 10.1186/s12906-025-04852-8
Assessing iIndications for herbal medicinal products: a comparative analysis of EMA monographs and database records
Abstract
Background: Adverse effects are common during cancer treatment and herbal medicinal products (HMPs) are one way to manage symptoms caused by conventional therapy.
Objectives: This assessment focused on comparing HMP indications listed in European Medicines Agency (EMA) monographs with findings in Medline and the Cochrane Library. The objective of this study was to investigate whether there is evidence that HMP indications may be transferred from non-cancer patients to cancer patients for the treatment of therapy-induced symptoms.
Methods: This study design included a comprehensive review of the relevant literature.
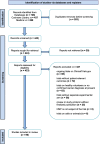
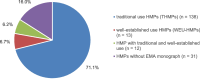
Results: The systematic literature search identified 96 clinical trials from a total of 726 records that met all the inclusion criteria. An analysis was performed on two groups: the EMA indication group vs. the non-EMA-indication group. The EMA indication group comprises trials whose endpoints align with the indications outlined in EMA monographs, representing a slight majority of 58.3% of all eligible clinical trials. In contrast, 41.7% of all studies were found to have non-EMA-indications, i.e. indications for cancer patients not listed in EMA monographs. Approximately 71.1% of all phytopharmaceuticals are approved as traditional use HMPs (THMPs).
Conclusion: The efforts of the EMA represent a fundamental step toward securing the quality of HMPs in the European Union (EU). However, a more systematic approach to conducting studies in such a tradition-bound field is required to generate evidence on HMPs. Given the absence of sufficient data, it is not possible to make a definitive statement on the transferability of HMP scopes listed in EMA monographs to the management of treatment-related symptoms in cancer patients.
Keywords: Cancer; EMA; Herbal medicinal product; Integrative medicine; List entry; Monograph; Phytopharmaceutical.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Direct-acting antivirals for chronic hepatitis C.Cochrane Database Syst Rev. 2017 Sep 18;9(9):CD012143. doi: 10.1002/14651858.CD012143.pub3. Cochrane Database Syst Rev. 2017. PMID: 28922704 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Herbal and dietary therapies for primary and secondary dysmenorrhoea.Cochrane Database Syst Rev. 2001;(3):CD002124. doi: 10.1002/14651858.CD002124. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2016 Mar 22;3:CD002124. doi: 10.1002/14651858.CD002124.pub2. PMID: 11687013 Updated.
References
-
- European Parliament CotEU. Directive 2004/24/EC 31 March 2004 amending, as regards traditional herbal medicinal products, Directive 2001/83/EC on the Community code relating to medicinal products for human use. Official J Eur Union. 2004. 85 - 90.
-
- European Parliament CotEU. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the community code relating to medicinal products for human use Official J Eur Union; 2001. 16.
-
- (HMPC) CoHMP. HMPC Rules of procedure Rev. 5. European Medicines Agency (EMA); 2021.
-
- Knöss W, Chinou I. Regulation of medicinal plants for public health – European community monographs on herbal substances. Planta Med. 2012;78(12):1311–6. - PubMed
-
- Chinou I. Monographs, list entries, public statements. J Ethnopharmacol. 2014;158:458–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous