Cross-sectional examination of characteristics of higher-dose buprenorphine prescriptions during the era of illicit fentanyl
- PMID: 40205451
- PMCID: PMC11980160
- DOI: 10.1186/s13722-025-00547-0
Cross-sectional examination of characteristics of higher-dose buprenorphine prescriptions during the era of illicit fentanyl
Abstract
Background: In response to greater illicit fentanyl use, buprenorphine daily doses exceeding the FDA's recommended target daily dose (16 mg) and maximum suggested daily dose (24 mg) may provide better outcomes, but little is known about higher dosage prescribing patterns. To better understand buprenorphine prescribing patterns, this manuscript examines the frequency and characteristics of dispensed buprenorphine of ≤ 16mg, > 16-24 mg, and > 24 mg daily dose.
Methods: We used IQVIA data to conduct a cross-sectional study of opioid use disorder-indicated buprenorphine prescriptions dispensed at retail pharmacies January 2019 - December 2020; categorized prescriptions as ≤ 16mg, > 16 to 24 mg, and > 24 mg daily dose; and examined overall rates and rates by patient, insurer and county characteristics, and prescriber specialty. We categorized buprenorphine prescriptions by patient sex, age cohort, primary payment source, and prescriber specialty and state and conducted univariate and bivariate analyses of buprenorphine daily dose categories overall and among clinicians frequently prescribing buprenorphine at the highest doses, > 24 mg.
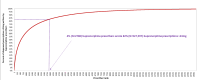
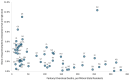
Results: Approximately 19.5% (n = 5,568,964) of the 28 million buprenorphine prescriptions from 68,898 clinicians were > 16-24 mg; 2% (n = 641,390) were > 24 mg. Approximately 26% (n = 17,939) of clinicians wrote at least one prescription > 24 mg; 2,780 clinicians (4% of buprenorphine prescribers) were responsible for 82.2% (n = 527,597) of dispensed prescriptions > 24 mg. 28% of prescriptions > 24 mg written by these prescribers were cash-pay, 12.5% covered by Medicaid, and 6.7% covered by Medicare. There was no correlation between state fentanyl overdose rate and buprenorphine prescriptions > 24 mg per 1,000,000 residents.
Conclusions: In 2019-2020, fewer than 3% of dispensed buprenorphine prescriptions exceeded the FDA suggested maximum of 24 mg daily dose; 80% of the prescriptions for a > 24 mg daily dose were written by 4% of buprenorphine prescribers. As clinicians and policymakers pay greater attention to ensuring individuals are receiving buprenorphine dosages adequate to effectively treat their opioid use disorder, the recently revised FDA recommendations may encourage such behavior. Additionally, disproportionate reliance on cash payment for higher daily doses suggests public and private insurers could facilitate access to such treatment when appropriate.
Keywords: Buprenorphine; Insurance; Opioid use disorder.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Ethics approval: The corresponding author’s Institutional Review Board approved the study with a waiver of consent. Consent for publication: N/A. Conflict of interest: The authors have no conflicts of interest to disclose. The manuscript represents valid work and that neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere.
Figures

References
-
- National Center for Health Statistics. Multiple Cause of Death 1999–2021. CDC WONDER Online Database. 2023. https://wonder.cdc.gov/mcd.html. Accessed 22 January 2024.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

