AGE induced macrophage-derived exosomes induce endothelial dysfunction in diabetes via miR-22-5p/FOXP1
- PMID: 40205587
- PMCID: PMC11983961
- DOI: 10.1186/s12933-025-02715-7
AGE induced macrophage-derived exosomes induce endothelial dysfunction in diabetes via miR-22-5p/FOXP1
Abstract
Background: Endothelial dysfunction is a pivotal contributor to cardiovascular complications in individuals with diabetes. However, the precise role of macrophages and their exosomes in the diabetic milieu remains elusive.
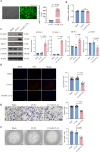
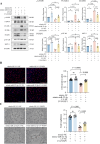
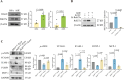
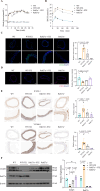
Methods: Exosomes (Exos) were isolated from the supernatants of macrophages treated with advanced glycation end products (AGE) or bovine serum albumin (BSA) using ultracentrifugation. Following coculture with AGE-Exos or BSA-Exos, human umbilical vein endothelial cells (HUVECs) were subjected to CCK-8, EdU, cell migration, monocyte adhesion, and tube formation assays. ELISA and Western blotting were employed to assess inflammatory cytokine release and protein expression levels in HUVECs. The miRNA expression profiles of AGE-Exos and BSA-Exos were analysed using miRNA arrays. Potential targets of miR-22-5p were predicted via miRNA databases and validated through RT‒qPCR, dual-luciferase reporter assays, and rescue experiments. Furthermore, a Rab27a knockout mouse model of type 2 diabetes mellitus (T2DM) was established by intraperitoneal injection of Streptozotocin. Aortic tissues were analysed via immunofluorescence for CD63 and CD31 expression, immunohistochemistry for VCAM-1 and ICAM-1 expression, and Western blotting for FOXP1 expression.
Results: AGE stimulation increased the secretion of exosomes from macrophages. Compared with BSA-Exos, AGE-Exos significantly impaired endothelial cell proliferation, migration, and tube formation capabilities while increasing monocyte adhesion and proinflammatory cytokine release without affecting cell viability. miR-22-5p was enriched in AGE-Exos, which were subsequently transferred to HUVECs, specifically targeting FOXP1, resulting in endothelial dysfunction. Overexpression of miR-22-5p in HUVECs using lentiviral vectors recapitulated the inflammatory effects observed with AGE-Exos, whereas anti-miR-22-5p conferred protective effects. Rab27a knockout significantly reduced exosome accumulation in T2DM model mouse aortic tissues, alleviating endothelial discontinuity, downregulating VCAM-1 and ICAM-1 expression, and upregulating FOXP1 expression.
Conclusions: AGE-induced release of macrophage-derived exosomes may partially depend on Rab27a transport, which delivers miR-22-5p to ECs. This miR-22-5p targets FOXP1 in ECs, leading to inflammation and resulting in endothelial dysfunction that accelerates the development of diabetic vascular lesions.
Keywords: Diabetes; Endotheliocytes; Exosomes; Inflammation; Macrophage; microRNAs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Our study was permitted by the Animal Ethics and the Use Committee of the Second Afliated Hospital of Guangzhou Medical University (A2019-027). All routine progresses were conducted in conformity to the Guide for the Use of Laboratory Animals. Consent for publication: All authors consent for the publication of this study. Competing interests: The authors declare no competing interests.
Figures







Similar articles
-
M1 macrophage-derived exosomes impair beta cell insulin secretion via miR-212-5p by targeting SIRT2 and inhibiting Akt/GSK-3β/β-catenin pathway in mice.Diabetologia. 2021 Sep;64(9):2037-2051. doi: 10.1007/s00125-021-05489-1. Epub 2021 Jun 11. Diabetologia. 2021. PMID: 34117507
-
Exosomal miR-423-5p Derived from Mineralized Osteoblasts Promotes Angiogenesis of Endothelial Cells by Targeting CXCL10.Front Biosci (Landmark Ed). 2024 Aug 9;29(8):278. doi: 10.31083/j.fbl2908278. Front Biosci (Landmark Ed). 2024. PMID: 39206914
-
Inhibiting Rab27a in renal tubular epithelial cells attenuates the inflammation of diabetic kidney disease through the miR-26a-5p/CHAC1/NF-kB pathway.Life Sci. 2020 Nov 15;261:118347. doi: 10.1016/j.lfs.2020.118347. Epub 2020 Aug 25. Life Sci. 2020. PMID: 32853650
-
Macrophage-driven exosomes regulate the progression of cardiovascular disease.Front Pharmacol. 2025 Apr 30;16:1563800. doi: 10.3389/fphar.2025.1563800. eCollection 2025. Front Pharmacol. 2025. PMID: 40371346 Free PMC article. Review.
-
Effects and mechanisms of exosomes in microenvironment angiogenesis in breast cancer: An updated review.Oncol Res. 2025 May 29;33(6):1323-1334. doi: 10.32604/or.2024.059113. eCollection 2025. Oncol Res. 2025. PMID: 40486870 Free PMC article. Review.
Cited by
-
Exo-hydrogel therapy: a revolutionary approach to managing diabetic complications.J Nanobiotechnology. 2025 Aug 11;23(1):558. doi: 10.1186/s12951-025-03621-6. J Nanobiotechnology. 2025. PMID: 40790200 Free PMC article. Review.
References
-
- Sardu C, Paolisso P, Sacra C, Mauro C, Minicucci F, Portoghese M, et al. Effects of metformin therapy on coronary endothelial dysfunction in patients with prediabetes with stable angina and nonobstructive coronary artery stenosis: the CODYCE multicenter prospective study. Diabetes Care. 2019;42(10):1946–55. - PubMed
-
- Banovic M, Athithan L, McCann GP. Aortic stenosis and diabetes mellitus: an ominous combination. Diab Vasc Dis Res. 2019;16(4):310–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

