Real-World Effectiveness and Safety of Mirikizumab Induction Therapy in Patients With Ulcerative Colitis: A Multicentre Retrospective Observational Study
- PMID: 40205711
- PMCID: PMC12107223
- DOI: 10.1111/apt.70140
Real-World Effectiveness and Safety of Mirikizumab Induction Therapy in Patients With Ulcerative Colitis: A Multicentre Retrospective Observational Study
Abstract
Background: In randomised controlled trials, mirikizumab achieved clinical remission and improved outcomes of patients with moderate to severe ulcerative colitis (UC). However, there is currently no real-world evidence for mirikizumab.
Aim: To evaluate the real-world effectiveness and safety of mirikizumab.
Methods: In a retrospective cohort study among three facilities, we included patients with UC who first received mirikizumab between June 2023 and April 2024. The primary outcome was the change in the partial Mayo score (PMS) from week 0 to 12. Secondary outcomes included changes in serum C-reactive protein (CRP) and leucine-rich α2-glycoprotein (LRG) levels from week 0 to 12; clinical remission rate (PMS < 2 with rectal bleeding subscore of 0), CRP remission rate (< 3.0 mg/L), and LRG remission rate (< 12.7 μg/mL) at week 12; and adverse events during induction therapy.
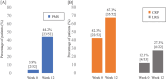
Results: We included 52 patients. Median (interquartile range) PMS decreased from week 0 to 12 (5 [3-6] to 2 [0-3], p < 0.001). CRP and LRG levels also decreased (CRP: 3.8 [0.9-7.3] to 1.8 [0.5-4.0] mg/L, p = 0.015; LRG: 20.1 [16.3-23.2] to 15.9 [12.8-23.2] μg/mL, p = 0.014). Rates of clinical remission, CRP remission, and LRG remission at week 12 were 44.2%, 67.3%, and 27.3%, respectively. There were no adverse events leading to permanent discontinuation of mirikizumab or death.
Conclusion: This real-world study demonstrated the short-term effectiveness and safety of mirikizumab in patients with UC.
Keywords: IL‐23; mirikizumab; ulcerative colitis; ustekinumab.
© 2025 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
Akira Nogami has served as speaker of AbbVie GK. Masataka Igeta has received advisor fees from Emol Inc. and Ono, Santen, and Takeda Pharmaceutical. Taku Kobayashi served as an advisory board member, consultant, or speaker for AbbVie, Alfresa Pharma, Alimentiv, Bristol Myers Squibb, Celltrion, Covidien, EA Pharma, Eli Lilly, Ferring Pharmaceuticals, Galapagos, Gilead Sciences, Janssen Pharmaceuticals, JIMRO, Kissei Pharmaceutical, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Nippon Kayaku, Pfizer, Takeda, and Zeria Pharmaceutical, and has received research funding from AbbVie, Alfresa Pharma, Bristol Myers Squibb, EA Pharma, Gilead Sciences, Helmsley Charitable Trust, Kyorin Pharmaceutical, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Pfizer, Sekisui Medical, Samsung, Takeda, and Zeria Pharmaceutical.
Shinichiro Shinzaki has received advisor fees from AbbVie GK, Janssen Pharmaceutical, and Takeda Pharmaceutical; and honoraria from AbbVie GK, Alfressa Pharma, AstraZeneca, EA Pharma, Eisai, Gilead Sciences, Kissei Pharmaceutical, Kyorin Pharmaceutical, Janssen Pharmaceutical, JIMRO, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Pfizer, Sekisui Medical, Takeda Pharmaceutical, and Zeria Pharmaceutical. The other authors declare no conflicts of interest.
Figures

References
-
- Ma C., Jairath V., Feagan B. G., et al., “Interpreting Modern Randomized Controlled Trials of Medical Therapy in Inflammatory Bowel Disease,” Nature Reviews. Gastroenterology & Hepatology 21, no. 11 (2024): 792–808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
