Elucidating the Role of Trem2 in Lipid Metabolism and Neuroinflammation
- PMID: 40205810
- PMCID: PMC11982525
- DOI: 10.1111/cns.70338
Elucidating the Role of Trem2 in Lipid Metabolism and Neuroinflammation
Abstract
Background: Alzheimer's disease (AD) is a neurodegenerative disorder characterized by cognitive impairment and neuroinflammation. Astrocytes play a key role in the neuroinflammatory environment of AD, especially through lipid metabolism regulation. However, the mechanisms by which astrocytes, particularly through the triggering receptor expressed on myeloid cells 2 (Trem2) receptor, contribute to lipid dysregulation and neuroinflammation in AD remain inadequately understood.
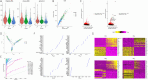
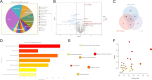
Methods: We employed an AD mouse model and integrated single-cell RNA sequencing (scRNA-seq), transcriptomics, and high-throughput metabolomics to analyze lipid metabolism and inflammatory profiles in astrocytes. Differential gene expression was further validated with the GEO database, and in vitro and in vivo experiments were conducted to assess the impact of Trem2 modulation on astrocytic inflammation and lipid composition.
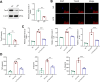
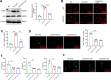
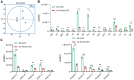
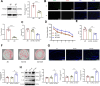
Results: Our findings demonstrate that Trem2 modulates lipid metabolism in astrocytes, affecting fatty acid and phospholipid pathways. In the AD model, Trem2 expression was suppressed, enhancing nuclear factor-κB (NF-κB) signaling and promoting the secretion of pro-inflammatory factors such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Trem2 overexpression reduced astrocytic inflammation and altered lipid composition, attenuating neuroinflammation both in vitro and in vivo. These results underscore Trem2's regulatory role in lipid metabolism and its significant impact on neuroinflammation in AD.
Conclusions: This study identifies Trem2 as a pivotal regulator of astrocytic lipid metabolism and neuroinflammation in AD, providing potential molecular targets for early intervention and therapeutic strategies aimed at mitigating AD progression.
Keywords: Alzheimer's disease; Trem2; astrocytes; lipid metabolism; neuroinflammation.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Integrative Metabolomics and Whole Transcriptome Sequencing Reveal Role for TREM2 in Metabolism Homeostasis in Alzheimer's Disease.Mol Neurobiol. 2024 Jul;61(7):4188-4202. doi: 10.1007/s12035-023-03840-8. Epub 2023 Dec 8. Mol Neurobiol. 2024. PMID: 38066402
-
Downregulation of TREM2 expression exacerbates neuroinflammatory responses through TLR4-mediated MAPK signaling pathway in a transgenic mouse model of Alzheimer's disease.Mol Immunol. 2022 Feb;142:22-36. doi: 10.1016/j.molimm.2021.12.018. Epub 2021 Dec 24. Mol Immunol. 2022. PMID: 34959070
-
A1 reactive astrocytes and a loss of TREM2 are associated with an early stage of pathology in a mouse model of cerebral amyloid angiopathy.J Neuroinflammation. 2020 Jul 25;17(1):223. doi: 10.1186/s12974-020-01900-7. J Neuroinflammation. 2020. PMID: 32711525 Free PMC article.
-
A systematic review of the role of TREM2 in Alzheimer's disease.Chin Med J (Engl). 2024 Jul 20;137(14):1684-1694. doi: 10.1097/CM9.0000000000003000. Epub 2024 Jun 24. Chin Med J (Engl). 2024. PMID: 38915213 Free PMC article.
-
TREM2 and the Progression of Alzheimer's Disease.Curr Neurovasc Res. 2017;14(2):177-183. doi: 10.2174/1567202614666170404165201. Curr Neurovasc Res. 2017. PMID: 28393702 Review.
Cited by
-
Systemic inflammation as a central player in the initiation and development of Alzheimer's disease.Immun Ageing. 2025 Aug 21;22(1):33. doi: 10.1186/s12979-025-00529-5. Immun Ageing. 2025. PMID: 40841660 Free PMC article. Review.
References
-
- Ali M. and Cascella M., “ICU Delirium,” in StatPearls (StatPearls Publishing, 2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

