Extracellular Vesicles Released From Skeletal Muscle Post-Chronic Contractile Activity Increase Mitochondrial Biogenesis in Recipient Myoblasts
- PMID: 40205946
- PMCID: PMC11982704
- DOI: 10.1002/jev2.70045
Extracellular Vesicles Released From Skeletal Muscle Post-Chronic Contractile Activity Increase Mitochondrial Biogenesis in Recipient Myoblasts
Abstract
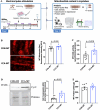
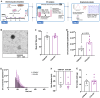
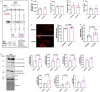
The effect of chronic contractile activity (CCA) on the biophysical properties and functional activity of skeletal muscle extracellular vesicles (Skm-EVs) is poorly understood due to challenges in distinguishing Skm-EVs originating from exercising muscle in vivo. To address this, myoblasts were differentiated into myotubes, and electrically paced (3 h/day, 4 days @ 14 V). CCA evoked an increase in mitochondrial biogenesis in stimulated versus non-stimulated (CON) myotubes as expected. EVs were isolated from conditioned media (CM) from control and stimulated myotubes using differential ultracentrifugation (dUC) and characterised biophysically using tunable resistive pulse sensing (TRPS, Exoid), TEM and western blotting. TEM images confirmed isolated round-shaped vesicles of about 30-150 nm with an intact lipid bilayer. EVs ranged from 98 to 138 nm in diameter, and the mean size was not altered by CCA. Zeta potential and total EV protein yield remained unchanged between groups, and total EV secretion increased after 4 days of CCA. Concomitant analysis of EVs after each day of CCA also demonstrated a progressive increase in CCA-EV concentration, whilst size and zeta potential remained unaltered, and EV protein yield increased in both CON-EVs and CCA groups. CCA-EVs were enriched with small-EVs versus CON-EVs, concomitant with higher expression of small-EV markers CD81, Tsg101 and HSP70. In whole cell lysates, CD63 and ApoA1 were reduced with CCA in myotubes, whereas CD81, Tsg101, Flotillin-1 and HSP70 levels remained unchanged. To evaluate the functional effect of EVs secreted post-CCA, we treated C2C12 myoblasts with all EVs isolated from CON or CCA myotubes after each day of stimulation, and measured cell count, cell viability, protein yield and mitochondrial biogenesis in recipient cells. There was no effect on cell count, viability and protein yield. Myoblasts treated with CCA-EVs exhibited increased mitochondrial biogenesis as indicated by enhanced MitoTracker Red staining, cytochrome c oxidase (COX) activity and protein expression of electron transport chain subunit, CIV-MTCO1. Further, CCA-EV treatment enhanced maximal oxygen consumption rates (OCR) in a dose-dependent manner, and ATP production in treated myoblasts. This increase in maximal OCR was abrogated when CCA-EVs pre-treated with proteinase K were co-cultured with myoblasts, indicating the pro-metabolic effect was likely mediated by transmembrane or peripheral membrane proteins in CCA-EVs. Our data highlight the novel effect of Skm-EVs isolated post-CCA in mediating pro-metabolic effects in recipient cells and thereby transmitting the effects associated with traditional exercise. Further investigation to interrogate the underlying mechanisms involved in downstream cellular metabolic adaptations is warranted.
Keywords: chronic contractile activity; differential ultracentrifugation; extracellular vesicles; mitochondrial biogenesis; myoblasts; myotubes; skeletal muscle cells.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

