Extracellular Vesicles Alter Trophoblast Function in Pregnancies Complicated by COVID-19
- PMID: 40205960
- PMCID: PMC11982706
- DOI: 10.1002/jev2.70051
Extracellular Vesicles Alter Trophoblast Function in Pregnancies Complicated by COVID-19
Abstract
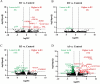
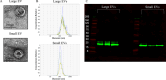
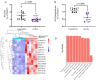
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and resulting coronavirus disease (COVID-19) cause placental dysfunction, which increases the risk of adverse pregnancy outcomes. While abnormal placental pathology resulting from COVID-19 is common, direct infection of the placenta is rare. This suggests that pathophysiology associated with maternal COVID-19, rather than direct placental infection, is responsible for placental dysfunction. We hypothesized that maternal circulating extracellular vesicles (EVs), altered by COVID-19 during pregnancy, contribute to placental dysfunction. To examine this hypothesis, we characterized circulating EVs from pregnancies complicated by COVID-19 and tested their effects on trophoblast cell physiology in vitro. Trophoblast exposure to EVs isolated from patients with an active infection (AI), but not controls, altered key trophoblast functions including hormone production and invasion. Thus, circulating EVs from participants with an AI, both symptomatic and asymptomatic cases, can disrupt vital trophoblast functions. EV cargo differed between participants with COVID-19, depending on the gestational timing of infection, and Controls, which may contribute to the disruption of the placental transcriptome and morphology. Our findings show that COVID-19 can have effects throughout pregnancy on circulating EVs, and circulating EVs are likely to participate in placental dysfunction induced by COVID-19.
Keywords: COVID‐19; extracellular vesicle cargo; extracellular vesicle origin; placental dysfunction; pregnancy.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Update of
-
Extracellular vesicles alter trophoblast function in pregnancies complicated by COVID-19.bioRxiv [Preprint]. 2024 May 23:2024.02.17.580824. doi: 10.1101/2024.02.17.580824. bioRxiv. 2024. Update in: J Extracell Vesicles. 2025 Apr;14(4):e70051. doi: 10.1002/jev2.70051. PMID: 38464046 Free PMC article. Updated. Preprint.
References
-
- Anton, L. , Brown A. G., Parry S., and Elovitz M. A.. 2012. “Lipopolysaccharide Induces Cytokine Production and Decreases Extravillous Trophoblast Invasion Through a Mitogen‐Activated Protein Kinase‐Mediated Pathway: Possible Mechanisms of First Trimester Placental Dysfunction.” Human Reproduction 27: 61–72. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

