Structure, function and evolution of the bacterial DinG-like proteins
- PMID: 40206346
- PMCID: PMC11981726
- DOI: 10.1016/j.csbj.2025.03.023
Structure, function and evolution of the bacterial DinG-like proteins
Abstract
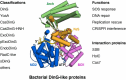
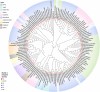
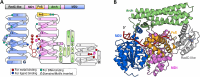
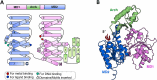
The damage-inducible G (DinG)-like proteins represent a widespread superfamily 2 (SF2) of DNA helicases, exhibiting remarkable diversity in domain architecture, substrate specificity, regulatory mechanisms, biological functions, interaction partners, and taxonomic distribution. Many characterized DinG-like proteins play critical roles in bacterial stress responses and immunity, including the SOS response, DNA repair, and phage interference. This review aims to provide a summary of bacterial DinG-like proteins, categorizing them into subgroups such as DinG, YoaA, CasDinG, CasDinG-HNH, ExoDinG, pExoDinG, EndoDinG, RadC-like DinG, sDinG, and others. This classification provides an analysis of sequence-structure-function relationships within this superfamily. Further sequence clustering revealed inter-cluster relationships and subgroup heterogeneity, suggesting potential functional divergence. Integrating sequence analysis, domain architecture, structural data, and genomic context enabled functional predictions for these DinG-like protein subgroups, shedding light on their evolutionary and biological significance.
Keywords: CRISPR interference; DNA repair; DinG; SOS response; Structure.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Genetic Analysis of DinG Family Helicase YoaA and Its Interaction with Replication Clamp Loader Protein HolC in Escherichia coli.J Bacteriol. 2021 Aug 20;203(18):e0022821. doi: 10.1128/JB.00228-21. Epub 2021 Aug 20. J Bacteriol. 2021. PMID: 34181484 Free PMC article.
-
The N-terminal domain of Type IV-A1 CRISPR-associated DinG is vulnerable to proteolysis.MicroPubl Biol. 2024 Jun 5;2024:10.17912/micropub.biology.001226. doi: 10.17912/micropub.biology.001226. eCollection 2024. MicroPubl Biol. 2024. PMID: 38911435 Free PMC article.
-
CasDinG is a 5'-3' dsDNA and RNA/DNA helicase with three accessory domains essential for type IV CRISPR immunity.Nucleic Acids Res. 2023 Aug 25;51(15):8115-8132. doi: 10.1093/nar/gkad546. Nucleic Acids Res. 2023. PMID: 37395408 Free PMC article.
-
For whom the bell tolls? DING proteins in health and disease.Cell Mol Life Sci. 2009 Jul;66(14):2205-18. doi: 10.1007/s00018-009-0006-6. Epub 2009 Mar 17. Cell Mol Life Sci. 2009. PMID: 19290474 Free PMC article. Review.
-
DING proteins: numerous functions, elusive genes, a potential for health.Cell Mol Life Sci. 2013 Sep;70(17):3045-56. doi: 10.1007/s00018-013-1377-2. Epub 2013 Jun 7. Cell Mol Life Sci. 2013. PMID: 23743708 Free PMC article. Review.
Cited by
-
Structural and functional investigation of DinG containing a 3'-5' exonuclease domain.mBio. 2025 Aug 13;16(8):e0088425. doi: 10.1128/mbio.00884-25. Epub 2025 Jun 30. mBio. 2025. PMID: 40586552 Free PMC article.
References
-
- White M.F. Structure, function and evolution of the XPD family of iron-sulfur-containing 5′-> 3′ DNA helicases. Biochem Soc Trans. 2009;37(Pt 3):547–551. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
