Diazomethyl-λ3-iodane meets aryne: dipolar cycloaddition and C-to-N iodane shift leading to indazolyl-λ3-iodanes
- PMID: 40206543
- PMCID: PMC11976445
- DOI: 10.1039/d5sc00266d
Diazomethyl-λ3-iodane meets aryne: dipolar cycloaddition and C-to-N iodane shift leading to indazolyl-λ3-iodanes
Abstract
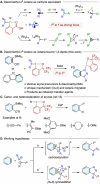
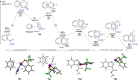
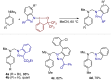
Diazomethyl-λ3-iodanes have recently emerged as carbyne equivalents in organic synthesis, enabling the construction of multi-substituted carbon centers through strategic sequential activation of the diazo and iodane functional groups. Distinct from such reaction modes, we report here on the reactivity of diazomethyl-λ3-iodanes as iodane-bound 1,3-dipoles toward arynes. Equipped with bis(trifluoromethyl)benzyl alcohol-based benziodoxole (BX) moiety, diazomethyl-λ3-iodanes undergo annulation with arynes generated from ortho-silylaryl triflates and cyclic diarylhalonium salts, resulting in indazolyl-λ3-iodanes through [3 + 2] cycloaddition and carbon-to-nitrogen iodane migration. DFT calculations reveal that diazomethyl-BX prefers [3 + 2] cycloaddition with aryne over aryne insertion into the carbon-iodine(iii) bond (carboiodanation) and that the subsequent iodane migration proceeds through two consecutive 1,5-iodane shifts. The utility of these indazolyl-BXs as indazole-transfer agents has been demonstrated by α-functionalization of N,N-dimethylaniline derivatives.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Yoshimura A. Zhdankin V. V. Chem. Rev. 2016;116:3328–3435. doi: 10.1021/acs.chemrev.5b00547. - DOI - PubMed
- Hari D. P. Caramenti P. Waser J. Acc. Chem. Res. 2018;51:3212–3225. doi: 10.1021/acs.accounts.8b00468. - DOI - PubMed
- Charpentier J. Fruh N. Togni A. Chem. Rev. 2015;115:650–682. doi: 10.1021/cr500223h. - DOI - PubMed
- Yoshimura A. Zhdankin V. V. Chem. Rev. 2024;124:11108–11186. doi: 10.1021/acs.chemrev.4c00303. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

