Continuous Improvement of Digital Health Applications Linked to Real-World Performance Monitoring: Safe Moving Targets?
- PMID: 40206630
- PMCID: PMC11975726
- DOI: 10.1016/j.mcpdig.2023.05.010
Continuous Improvement of Digital Health Applications Linked to Real-World Performance Monitoring: Safe Moving Targets?
Abstract
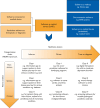
Real-time high-quality data on the performance of digital health applications is needed for feedback-led optimization and to ensure safety and performance, particularly if they will have on-market updates. Developers must verify that applications accurately and consistently fulfill their intended purpose in real-world use. In particular, new thinking from regulators recognizes the importance of monitoring real-world performance. It is acknowledged that real-world data can deliver information from wider patient populations than are generally included in controlled studies, and in certain circumstances, this can enable extensions of the application's intended purpose. Proactive postmarket surveillance surveys are an important source of real-world data that are distinct from clinical investigations but may vary in quality and, if inappropriately designed, can be subject to uncontrolled bias. We aimed to describe the practice of real-world data gathering through patient-reported and clinician-reported outcomes and high-quality surveys and identify challenges, uncertainties, and health policy gaps.
© 2023 The Authors.
Conflict of interest statement
Dr Gilbert has or has had consulting relationships with Una Health GmbH, Lindus Health Ltd, FLO Ltd, Thymia Ltd, and Ada Health GmbH and holds share options in Ada Health GmbH. Dr Pimenta is an employee of Ada Health GmbH. Dr Stratton-Powell is an employee of RQM+. Dr Welzel declares no competing interests. Dr Melvin is an unpaid advisory board member of Pumpinheart Ltd; previously, a senior medical officer in medical devices at the Health Products Regulatory Authority, Ireland; and a previous co-chair of the Clinical Investigation and Evaluation Working Group of the European Commission.
Figures


References
-
- IMDRF Software as a Medical Device (SaMD) Working Group . IMDRF; 2014. ‘Software as a Medical Device’: Possible Framework for Risk Categorization and Corresponding Considerations.
-
- Medical Device Coordination Group. Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746–IVDR. MDCG; 2019. md_mdcg_2019_11_guidance_qualification_classification_software_en_0.pdf.
Publication types
LinkOut - more resources
Full Text Sources

