AAV-mediated GBA1 and GDNF rescue neurological defects in a murine model of neuronopathic Gaucher disease
- PMID: 40206656
- PMCID: PMC11979523
- DOI: 10.1016/j.omtn.2025.102506
AAV-mediated GBA1 and GDNF rescue neurological defects in a murine model of neuronopathic Gaucher disease
Abstract
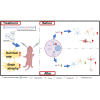
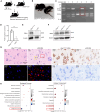
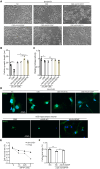
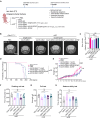
Neuropathic Gaucher disease (nGD) is a life-threatening disease that progresses rapidly and is caused by a glucosylceramidase beta 1 (GBA1) mutation, which encodes the lysosomal hydrolase β-glucocerebrosidase (GCase). Nerve damage in nGD, associated with stunted growth and development, arises from the degeneration and death of nervous system cells, which is often irreversible. Approved therapies effectively reduce the substrate burden outside the central nervous system (CNS) through augmenting mutant enzyme activity with pharmacologic recombinant GCase or by inhibiting glucocerebroside synthesis. However, these therapies do not provide neuroprotection. In this study, we developed a novel double-gene therapy based on adeno-associated virus (AAV), AAV9-GBA1-GDNF, which stably expresses human GBA1 and glial derived neurotrophic factor (GDNF) over the long term. Pathological, molecular, and proteomic tests in the nGD model confirmed that the early stages of the disease are characterized by GBA1 deficiency, loss of neuronal function, and even neuronal death. After treatment with AAV9-GBA1-GDNF, the lifespan of nGD mice was extended, and weight, brain development, and motor ability were recovered. Additionally, GBA1 and GDNF additively prevented irreversible neuronal death by activating the AKT/GSK3β pathway. These findings offer potential therapeutic strategies for nGD and other neurodegenerative diseases associated with lysosomal dysfunction.
Keywords: AAV; Dual targets; GBA1 related PD; GD; GDNF; MT; Neurodegeneration; Neurotrophic factors; Oligonucleotides; Therapies and Applications.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
PGRN deficiency exacerbates, whereas a brain penetrant PGRN derivative protects, GBA1 mutation-associated pathologies and diseases.Proc Natl Acad Sci U S A. 2023 Jan 3;120(1):e2210442120. doi: 10.1073/pnas.2210442120. Epub 2022 Dec 27. Proc Natl Acad Sci U S A. 2023. PMID: 36574647 Free PMC article.
-
Elevated Dkk1 Mediates Downregulation of the Canonical Wnt Pathway and Lysosomal Loss in an iPSC Model of Neuronopathic Gaucher Disease.Biomolecules. 2020 Dec 3;10(12):1630. doi: 10.3390/biom10121630. Biomolecules. 2020. PMID: 33287247 Free PMC article.
-
Neuronopathic Gaucher disease: Beyond lysosomal dysfunction.Front Mol Neurosci. 2022 Aug 3;15:934820. doi: 10.3389/fnmol.2022.934820. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35992201 Free PMC article. Review.
-
iPSC-derived neural precursor cells engineering GBA1 recovers acid β-glucosidase deficiency and diminishes α-synuclein and neuropathology.Mol Ther Methods Clin Dev. 2023 Mar 15;29:185-201. doi: 10.1016/j.omtm.2023.03.007. eCollection 2023 Jun 8. Mol Ther Methods Clin Dev. 2023. PMID: 37063480 Free PMC article.
-
Targeting the GBA1 pathway to slow Parkinson disease: Insights into clinical aspects, pathogenic mechanisms and new therapeutic avenues.Pharmacol Ther. 2023 Jun;246:108419. doi: 10.1016/j.pharmthera.2023.108419. Epub 2023 Apr 19. Pharmacol Ther. 2023. PMID: 37080432 Review.
Cited by
-
Decoding Parkinson's Disease: The interplay of cell death pathways, oxidative stress, and therapeutic innovations.Redox Biol. 2025 Jul 23;85:103787. doi: 10.1016/j.redox.2025.103787. Online ahead of print. Redox Biol. 2025. PMID: 40712453 Free PMC article. Review.
References
-
- Biegstraaten M., van Schaik I.N., Aerts J.M.F.G., Hollak C.E.M., Hollak C.E. 'Non-neuronopathic' Gaucher disease reconsidered. Prevalence of neurological manifestations in a Dutch cohort of type I Gaucher disease patients and a systematic review of the literature. J. Inherit. Metab. Dis. 2008;31:337–349. - PubMed
LinkOut - more resources
Full Text Sources

