Neutrophils in Rheumatoid Arthritis Synovium: Implications on Disease Activity and Inflammation State
- PMID: 40206809
- PMCID: PMC11980796
- DOI: 10.2147/JIR.S503144
Neutrophils in Rheumatoid Arthritis Synovium: Implications on Disease Activity and Inflammation State
Abstract
Background: Rheumatoid arthritis (RA) is characterized by chronic synovial inflammation driven by immune cell infiltration. While neutrophils have traditionally been associated with acute inflammation, emerging evidence suggests their significant role in chronic RA synovitis. Synovial pathology reports from our center reveal lymphocyte-predominant infiltration in most RA cases, with synovial neutrophils (SNs) observed in only 30% of patients. This finding suggests that neutrophil involvement in RA pathogenesis is not universal but subtype-specific, potentially linked to distinct clinical phenotypes.
Methods: We performed a retrospective analysis of synovial pathology and clinical data from 55 RA patients collected during 2023. Using both Hematoxylin-Eosin (H&E) staining and single-cell RNA sequencing, we analyzed the synovial tissue samples. Based on neutrophil counts, patients were classified into two groups: neutrophil-absent (<10 neutrophils) and neutrophil-present (≥10 neutrophils).
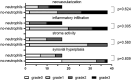
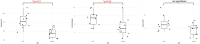
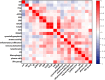
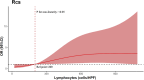
Results: In this cohort of 55 RA patients, the synovial neutrophil (SN) group demonstrated significantly elevated disease activity markers, including Disease Activity Score in 28 joints based on C-reactive protein (DAS28-CRP), swollen joint count (SJC28), Visual Analog Scale (VAS) pain scores, and tender joint count (TJC28) (p < 0.05 for all parameters). Synovial inflammatory infiltration and neovascularization were markedly increased in the SNs group (P < 0.05). Patients with SNs maintained higher disease activity and showed poorer therapeutic responses despite treatment with methotrexate and targeted biologics (TNF inhibitors, IL-6 inhibitors, or JAK inhibitors). Analysis revealed a positive correlation between lymphocyte and neutrophil counts, while multivariate analysis identified DAS28-CRP, synovial inflammation, and CD3+/CD68+ cell counts as predictors of SN infiltration. Single-cell RNA sequencing confirmed their significant presence in synovial tissue, supporting neutrophils' role in refractory disease.
Conclusion: Elevated neutrophil presence in RA synovium correlates with heightened clinical disease activity and an exacerbated inflammatory state. These findings underscore the potential significance of SNs in the pathology of RA.
Keywords: disease activity; immune cell infiltration; rheumatoid arthritis; synovial biopsy; synovial neutrophils.
© 2025 Deng et al.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Vascular synovial phenotype indicates poor response to JAK inhibitors in rheumatoid arthritis patients: a pilot study.PeerJ. 2024 Nov 29;12:e18631. doi: 10.7717/peerj.18631. eCollection 2024. PeerJ. 2024. PMID: 39624123 Free PMC article.
-
Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy.Arthritis Res Ther. 2005;7(2):R359-69. doi: 10.1186/ar1501. Epub 2005 Jan 21. Arthritis Res Ther. 2005. PMID: 15743484 Free PMC article.
-
Immunophenotypic Landscape of synovial tissue in rheumatoid arthritis: Insights from ACPA status.Heliyon. 2024 Jul 4;10(13):e34088. doi: 10.1016/j.heliyon.2024.e34088. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39055820 Free PMC article.
-
The clinical features of rheumatoid arthritis.Eur J Radiol. 1998 May;27 Suppl 1:S18-24. doi: 10.1016/s0720-048x(98)00038-2. Eur J Radiol. 1998. PMID: 9652497 Review.
-
New Developments in Transcriptomic Analysis of Synovial Tissue.Front Med (Lausanne). 2020 Jan 31;7:21. doi: 10.3389/fmed.2020.00021. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32083090 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

