Photochemical Manganese-Catalyzed [2 + 2 + 2] Cycloaddition Reactions
- PMID: 40207070
- PMCID: PMC11976702
- DOI: 10.1021/acscatal.5c00349
Photochemical Manganese-Catalyzed [2 + 2 + 2] Cycloaddition Reactions
Abstract
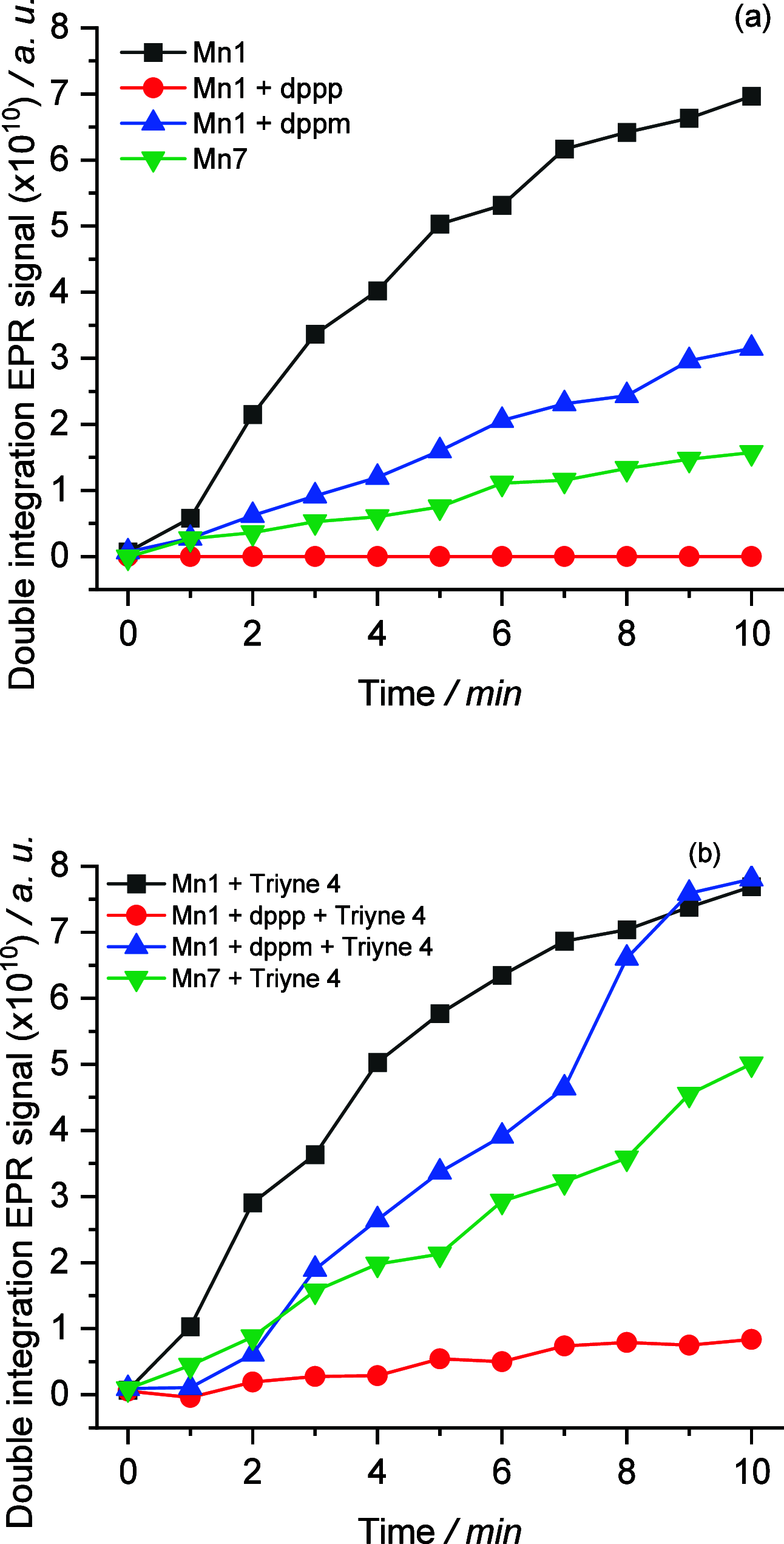
We report the cyclotrimerization reactions of triynes using Mn(I) complexes derived from MnBr(CO)5 and phosphine ligands, such as 1,1-bis(diphenylphosphino)methane (dppm). These reactions are driven by irradiation under mild conditions (30-80 °C) without the need of additional photoinitiators. Our catalytic screening revealed that counteranions and ligands significantly influence the process. This method accommodates a broad range of functionalities in the substrates, including alkyl, aryl, Bpin, SiMe3, GeEt3, PPh2, pyridyl, and thienyl moieties, without notable interference in the transformation. Additionally, this method enables reactions with oligoalkynes-like (un)substituted hexaynes, producing 2-fold cyclization products in very good yields. Under stoichiometric conditions, the cyclization of diynes with phosphaalkynes results in the unique photochemical synthesis of phosphinines. Experimental and theoretical mechanistic studies indicate that the dissociation of the diphosphine ligand precedes the involvement of the Mn carbonyl species in the catalytic cycle. The ligand plays a crucial role in stabilizing the catalyst during the catalytic transformation and preventing the formation of unreactive cluster species.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Vollhardt K. P. C. Transition-metal-catalyzed acetylene cyclizations in organic synthesis. Acc. Chem. Res. 1977, 10, 1–8. 10.1021/ar50109a001. - DOI
-
- Cioslowski J.; Liu G.; Moncrieff D. The concerted trimerization of ethyne to benzene revisited. Chem. Phys. Lett. 2000, 316, 536–540. 10.1016/S0009-2614(99)01319-6. - DOI
-
- Tanaka K.Transition-Metal-Mediated Aromatic Ring Construction; John Wiley & Sons, 2013.
-
- Hapke M.; Weding N.; Kral K.. Cyclotrimerization Reactions of Alkynes. In Applied Homogeneous Catalysis with Organometallic Compounds, Vol. 1, 3rd ed (Eds.: Cornils B.; Herrmann W. A.; Beller M.; Paciello R.); Wiley-VCH: Weinheim, 2018; pp 375–410.
-
- Yamamoto Y. Recent advances in intramolecular alkyne cyclotrimerization and its applications. Curr. Org. Chem. 2005, 9, 503–519. 10.2174/1385272053544399. - DOI
- Chopade P. R.; Louie J. [2 + 2 + 2] Cycloaddition reactions catalyzed by transition metal complexes. Adv. Synth. Catal. 2006, 348, 2307–2327. 10.1002/adsc.200600325. - DOI
- Gandon V.; Aubert C.; Malacria M. Recent progress in cobalt-mediated [2 + 2 + 2] cycloaddition reactions. Chem. Commun. 2006, 2209–2217. 10.1039/b517696b. - DOI - PubMed
- Agenet N.; Buisine O.; Slowinski F.; Gandon V.; Aubert C.; Malacria M. Cotrimerizations of acetylenic compounds. Org. React. 2007, 68, 1–302. 10.1002/0471264180.or068.01. - DOI
- Tanaka K. Cationic Rhodium(I)/BINAP-Type bisphosphine complexes: versatile new catalysts for highly chemo-, regio-, and enantioselective [2 + 2 + 2] cycloadditions. Synlett 2007, 2007, 1977–1993. 10.1055/s-2007-984541. - DOI
- Scheuermann C. J.; Ward B. D. Selected recent developments in organo-cobalt chemistry. New J. Chem. 2008, 32, 1850–1880. 10.1039/b802689k. - DOI
- Galan B. R.; Rovis T. Beyond Reppe: Building Substituted Arenes by Cycloadditions of [2 + 2 + 2]-Cycloadditions of Alkynes. Angew. Chem., Int. Ed. 2009, 48, 2830–2834. 10.1002/anie.200804651. - DOI - PMC - PubMed
- Tanaka K. Transition-metal-catalyzed enantioselective [2 + 2 + 2] cycloadditions for the synthesis of axially chiral biaryls. Chem. - Asian J. 2009, 4, 508–518. 10.1002/asia.200800378. - DOI - PubMed
- Domínguez G.; Pérez-Castells J. Recent advances in [2 + 2 + 2] cycloaddition reactions. Chem. Soc. Rev. 2011, 40, 3430–3444. 10.1039/c1cs15029d. - DOI - PubMed
- Hua R.; Abrenica M. V. A.; Wang P. Cycloaddition of Alkynes: Atom-economic protocols for constructing Six-Membered Cycles. Wang. Curr. Org. Chem. 2011, 15, 712–729. 10.2174/138527211794519023. - DOI
- Tanaka K.; Shibata Y. Rhodium-Catalyzed [2 + 2 + 2] cycloaddition of alkynes for the synthesis of substituted benzenes: catalysts, reaction scope, and synthetic applications. Synthesis 2012, 44, 323–350. 10.1055/s-0031-1289665. - DOI
- Ruijter E.; Broere D. Recent Advances in Transition-Metal-Catalyzed [2 + 2 + 2]-Cyclo(co)trimerization Reactions. Synthesis 2012, 44, 2639–2672. 10.1055/s-0032-1316757. - DOI
- Okamoto S.; Sugiyama Y.-K. From the development of catalysts for alkyne and Alkyne-Nitrile [2 + 2 + 2] cycloaddition reactions to their use in polymerization reactions. Synlett 2013, 24, 1044–1060. 10.1055/s-0032-1316913. - DOI
- Amatore M.; Aubert C. Recent advances in stereoselective [2 + 2 + 2] cycloadditions. C. Aubert. Eur. J. Org. Chem. 2015, 2015, 265–286. 10.1002/ejoc.201403012. - DOI
- Thakur A.; Louie J. Advances in Nickel-Catalyzed cycloaddition reactions to construct carbocycles and heterocycles. Acc. Chem. Res. 2015, 48, 2354–2365. 10.1021/acs.accounts.5b00054. - DOI - PMC - PubMed
- Domínguez G.; Pérez-Castells J. Alkenes in [2 + 2 + 2] cycloadditions. Chem. - Eur. J. 2016, 22, 6720–6739. 10.1002/chem.201504987. - DOI - PubMed
- Hilt G.; Röse P. Cobalt-Catalysed Bond Formation Reactions; Part 2. Synthesis 2016, 48, 463–492. 10.1055/s-0035-1560378. - DOI
- Kotha S.; Lahiri K.; Sreevani G. Design and Synthesis of Aromatics through [2 + 2 + 2] Cyclotrimerization. Synlett 2018, 29, 2342–2361. 10.1055/s-0037-1609584. - DOI
- Staudaher N. D.; Stolley R. M.; Louie J. [2 + 2 + 2] Cycloadditions of Alkynes with Heterocumulenes and Nitriles. Organic Reactions 2019, 1–202. 10.1002/0471264180.or097.01. - DOI
- Ratovelomanana-Vidal V.; Matton P.; Huvelle S.; Haddad M.; Phansavath P. Recent progress in metal-catalyzed [2 + 2 + 2] cycloaddition reactions. Synthesis 2022, 54, 4–32. 10.1055/s-0040-1719831. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
