Integrated primary and secondary care optimizes the management of people with CKD-the LUCID project
- PMID: 40207101
- PMCID: PMC11980979
- DOI: 10.1093/ckj/sfaf049
Integrated primary and secondary care optimizes the management of people with CKD-the LUCID project
Abstract
Background: Early diagnosis, risk stratification and medication optimization are essential to improve the management of chronic kidney disease (CKD) and other long-term conditions. The introduction of Integrated Care Systems (ICS) in England provides the opportunity to revolutionize the management of these conditions. Annual National Health Service kidney disease costs are ∼£6.4 billion.
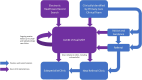
Methods: We designed, piloted and implemented at scale an ICS-level virtual care programme for CKD, the 'Leicester, Leicestershire, and Rutland Chronic Kidney Disease Integrated Care Delivery Project' (LUCID), based on the principles of patient and professional education, early disease identification, medicines optimization and disease surveillance.
Results: In April 2022, virtual multidisciplinary team (MDT) meetings were piloted in Leicester, Leicestershire and Rutland, UK. Since April 2023 virtual MDT meetings have been available to all general practices in Leicester, Leicestershire and Rutland, representing a population of approximately 1.2 million people. As of 31 March 2024, general practices representing an estimated population of 700 000 (58.3%) were participating in the LUCID programme. Some 1085 consultations took place for 821 patients, 590 (54.4%) of which were medicines optimization consultations.
Conclusions: LUCID may represent an efficient and cost-effective model to deliver patient and professional education, medicine optimization and risk stratification for people living with CKD at an ICS-wide population level. This model may be adaptable for other long-term physical and mental health conditions.
Keywords: CKD; CVD; KFRE; integrated care; medicine optimisation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
From April 2023 to November 2024 LUCID was part funded as part of a Joint Working Initiative with AstraZeneca UK. Major, Lakhani, Baines, Graham-Brown, Burton declare speaker honorarium from AstraZeneca. Jinadu and Steiner declare support from AstraZeneca for travelling to meetings. Major, Lakhani, Graham-Brown and Burton declare speaker honoraria from Boehringer Ingelheim and Bayer. Baines declares fees from George Clinical. Lakhani also declares speaker honoraria from Amarin, Eli Lilly, Novartis, Novo Nordisk, Daiichi-Sankyo and Viatris.
Figures

References
-
- National Institute for Health and Care Excellence . Chronic Kidney Disease: Assessment and Management NG203 [Internet]. London: NICE. 2021; [published 25 August 2021; cited 15 August 2024]. Available from: https://www.nice.org.uk/guidance/ng80 - PubMed
-
- National Institute for Health and Care Excellence . Dapagliflozin for Treating Chronic Kidney Disease TA775 [Internet]. London: NICE. 2022; [published 9th March 2022; cited 15 August 2024]. Available from: https://www.nice.org.uk/guidance/ta775 (15 August 2024, date last accessed). - PubMed
-
- National Institute for Health and Care Excellence . Empagliflozin for Treating Chronic Kidney Disease TA942 [Internet]. London: NICE. 2022; [published 20th December 2023; cited 15 August 2024]. Available from: https://www.nice.org.uk/guidance/ta942 (15 August 2024, date last accessed). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

