The efficacy and safety of PD-1/PD-L1 inhibitors in combination with chemotherapy as a first-line treatment for unresectable, locally advanced, HER2-negative gastric or gastroesophageal junction cancer: a meta-analysis of randomized controlled trials
- PMID: 40207218
- PMCID: PMC11979168
- DOI: 10.3389/fimmu.2025.1566939
The efficacy and safety of PD-1/PD-L1 inhibitors in combination with chemotherapy as a first-line treatment for unresectable, locally advanced, HER2-negative gastric or gastroesophageal junction cancer: a meta-analysis of randomized controlled trials
Abstract
Background: Immune checkpoint inhibitors (ICIs) plus fluorouracil-based chemotherapy (Chemo) have been approved as an initial treatment strategy for metastatic or recurrent human epidermal growth factor receptor 2 (HER2)-negative gastric cancer (GC) or gastroesophageal junction cancer (GEJC). However, since programmed cell death protein-1 (PD-1) or its ligand 1 (PD-L1) inhibitors have just recently been investigated for the treatment of unresectable GC/GEJC, there is ongoing debate regarding their safety and effectiveness for prespecified subgroups. The purpose of this research is to establish a foundation toward stratified decision-making by methodically assessing the merits and drawbacks of PD-1/PD-L1 inhibitors combined with chemo in the clinical utilization of advanced HER2-negative GC/GEJC according to certain prominent large-scale randomized controlled trials (RCTs). In addition, we limitedly explored the favorable short-term efficacy of PD-1/CTLA-4 bispecific antibodies for the above-mentioned tumors.
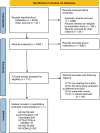
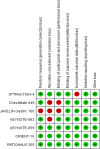
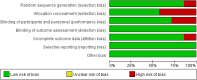
Methods: The researchers retrieved several databases, including PubMed, Embase, Web of Science, ClinicalTrials.gov, and the Cochrane Library, to collect all the relevant literature published since the establishment of the databases until October 30, 2024, and then screened to determine the qualified literature and extracted the relevant information. We only included RCTs for PD-1/PD-L1 inhibitors with or without chemo in advanced GC or GEJC. The primary endpoints were overall survival (OS), progression-free survival (PFS), and objective response rate (ORR). A subgroup analysis for the median overall survival (mOS) was conducted for the following variables: microsatellite instability (MSI) status, PD-L1 expression, combined positive scores (CPS), metastasis status, and primary tumor location. When moderate heterogeneity was found, a random-effect model was applied. The outcome indicators were then statistically analyzed, taking advantage of Review Manager 5.4. Hazard ratio (HR) and risk ratio (RR) were selected as the effect values for statistical analysis.
Results: A total of 7 eligible RCTs and 6537 participants were included in this meta-analysis. Combining PD-1/PD-L1 inhibitors with chemo significantly improved patients' OS compared with chemo alone, especially in the tumor cell PD-L1 expression ≥ 1% [HR = 0.62, 95% CI (0.48, 0.81); a p-value = 0.0004], PD-L1 CPS ≥ 10 [HR = 0.66, 95% CI (0.57, 0.77); a p-value < 0.00001], and MSI-H subgroups [HR = 0.40, 95% CI (0.28, 0.59); a p-value < 0.00001]. Moreover, distinct primary tumor location (GC or GEJC) and the presence of liver metastases could also benefit from the additive or sustained effect of anti-cancer chemo-immunotherapy.
Conclusion: For patients with advanced HER2-negative GC/GEJC, PD-1/PD-L1 inhibitors in combination with chemo have almost demonstrated consistent synergistic anti-tumor benefits to survival outcomes when compared to chemo alone. However, the subgroup analysis in this meta-study revealed that neither PD-L1 expression level nor MSI status could fully predict the efficacy of the dual treatment model but faced a higher possibility of serious treatment-related adverse events (sTRAEs), particularly in the synchronous therapy arm. Therefore, urging the need for more investigations into the development of collaborative prognostic forecasting models for achieving precise stratification, established harmonized testing standards and methods for PD-L1 expression and positivity, optimal CPS threshold for benefits, as well as alternative molecular biomarkers for the reason that certain indicators alone may not discriminate responders clearly. Lastly, dual anti-therapy might be a useful tactic for the population with low PD-L1 expression in the future.
Keywords: PD-1/PD-L1 inhibitors; advanced gastroesophageal cancer; chemotherapy; gastric adenocarcinoma; gastroesophageal adenocarcinoma; immune check points; meta-analysis; overall survival.
Copyright © 2025 Pu, Li, Zhang, Huang, Li, Jiang, Xu, Yi, Lan, Xiao, Chen and Jin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








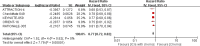




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

