Impact of lactate on immune cell function in the tumor microenvironment: mechanisms and therapeutic perspectives
- PMID: 40207222
- PMCID: PMC11979165
- DOI: 10.3389/fimmu.2025.1563303
Impact of lactate on immune cell function in the tumor microenvironment: mechanisms and therapeutic perspectives
Abstract
Lactate has emerged as a key regulator in the tumor microenvironment (TME), influencing both tumor progression and immune dynamics. As a byproduct of aerobic glycolysis, lactate satisfies the metabolic needs of proliferating tumor cells while reshaping the TME to facilitate immune evasion. Elevated lactate levels inhibit effector immune cells such as CD8+ T and natural killer cells, while supporting immunosuppressive cells, such as regulatory T cells and myeloid-derived suppressor cells, thus fostering an immunosuppressive environment. Lactate promotes epigenetic reprogramming, stabilizes hypoxia-inducible factor-1α, and activates nuclear factor kappa B, leading to further immunological dysfunction. In this review, we examined the role of lactate in metabolic reprogramming, immune suppression, and treatment resistance. We also discuss promising therapeutic strategies targeting lactate metabolism, including lactate dehydrogenase inhibitors, monocarboxylate transporter inhibitors, and TME neutralization methods, all of which can restore immune function and enhance immunotherapy outcomes. By highlighting recent advances, this review provides a theoretical foundation for integrating lactate-targeted therapies into clinical practice. We also highlight the potential synergy between these therapies and current immunotherapeutic strategies, providing new avenues for addressing TME-related challenges and improving outcomes for patients with cancer.
Keywords: immunosuppression; immunotherapy; lactate metabolism; targeted therapy; tumor microenvironment.
Copyright © 2025 Gu, Yang, Lai, Zhou, Tang, Hu and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
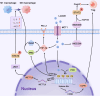
Figures

Similar articles
-
From metabolic byproduct to immune modulator: the role of lactate in tumor immune escape.Front Immunol. 2024 Nov 25;15:1492050. doi: 10.3389/fimmu.2024.1492050. eCollection 2024. Front Immunol. 2024. PMID: 39654883 Free PMC article. Review.
-
Lactate in the tumour microenvironment: From immune modulation to therapy.EBioMedicine. 2021 Nov;73:103627. doi: 10.1016/j.ebiom.2021.103627. Epub 2021 Oct 14. EBioMedicine. 2021. PMID: 34656878 Free PMC article. Review.
-
The oncogenic and clinical implications of lactate induced immunosuppression in the tumour microenvironment.Cancer Lett. 2021 Mar 1;500:75-86. doi: 10.1016/j.canlet.2020.12.021. Epub 2020 Dec 23. Cancer Lett. 2021. PMID: 33347908
-
Lactate-mediated metabolic reprogramming of tumor-associated macrophages: implications for tumor progression and therapeutic potential.Front Immunol. 2025 May 13;16:1573039. doi: 10.3389/fimmu.2025.1573039. eCollection 2025. Front Immunol. 2025. PMID: 40433363 Free PMC article. Review.
-
Research progress on the regulatory role of lactate and lactylation in tumor microenvironment.Biochim Biophys Acta Rev Cancer. 2025 Jul;1880(3):189339. doi: 10.1016/j.bbcan.2025.189339. Epub 2025 Apr 29. Biochim Biophys Acta Rev Cancer. 2025. PMID: 40311713 Review.
Cited by
-
Metabolic Reprogramming in Melanoma: An Epigenetic Point of View.Pharmaceuticals (Basel). 2025 Jun 6;18(6):853. doi: 10.3390/ph18060853. Pharmaceuticals (Basel). 2025. PMID: 40573249 Free PMC article. Review.
-
Role of Tumor Microenvironment in Prostate Cancer Immunometabolism.Biomolecules. 2025 Jun 6;15(6):826. doi: 10.3390/biom15060826. Biomolecules. 2025. PMID: 40563466 Free PMC article. Review.
-
Clinical application prospects of traditional Chinese medicine as adjuvant therapy for metabolic reprogramming in colorectal cancer.Front Immunol. 2025 Jul 15;16:1630279. doi: 10.3389/fimmu.2025.1630279. eCollection 2025. Front Immunol. 2025. PMID: 40735327 Free PMC article. Review.
-
Metabolic Interactions in the Tumor Microenvironment of Classical Hodgkin Lymphoma: Implications for Targeted Therapy.Int J Mol Sci. 2025 Aug 4;26(15):7508. doi: 10.3390/ijms26157508. Int J Mol Sci. 2025. PMID: 40806636 Free PMC article. Review.
-
Context Matters: Divergent Roles of Exercise-Induced and Tumor-Derived Lactate in Cancer.Biomolecules. 2025 Jul 14;15(7):1010. doi: 10.3390/biom15071010. Biomolecules. 2025. PMID: 40723880 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

