Unveiling the fungal frontier: mycological insights into inflammatory bowel disease
- PMID: 40207229
- PMCID: PMC11979276
- DOI: 10.3389/fimmu.2025.1551289
Unveiling the fungal frontier: mycological insights into inflammatory bowel disease
Abstract
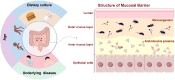
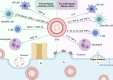
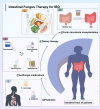
Inflammatory bowel disease (IBD) is a chronic recurrent gastrointestinal disease that seriously affects the quality of life of patients around the world. It is characterized by recurrent abdominal pain, diarrhea, and mucous bloody stools. There is an urgent need for more accurate diagnosis and effective treatment of IBD. Accumulated evidence suggests that gut microbiota plays an important role in the occurrence and development of gut inflammation. However, most studies on the role of gut microbiota in IBD have focused on bacteria, while fungal microorganisms have been neglected. Fungal dysbiosis can activate the host protective immune pathway related to the integrity of the epithelial barrier and release a variety of pro-inflammatory cytokines to trigger the inflammatory response. Dectin-1, CARD9, and IL-17 signaling pathways may be immune drivers of fungal dysbacteriosis in the development of IBD. In addition, fungal-bacterial interactions and fungal-derived metabolites also play an important role. Based on this information, we explored new strategies for IBD treatment targeting the intestinal fungal group and its metabolites, such as fungal probiotics, antifungal drugs, diet therapy, and fecal microbiota transplantation (FMT). This review aims to summarize the fungal dysbiosis and pathogenesis of IBD, and provide new insights and directions for further research in this emerging field.
Keywords: fungi; inflammatory bowel disease; microbiology; pathogenesis; treatment.
Copyright © 2025 Chen, Yi, Yi, Zhou, Song and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues.Gastroenterology. 2021 Jan;160(2):524-537. doi: 10.1053/j.gastro.2020.09.056. Epub 2020 Nov 27. Gastroenterology. 2021. PMID: 33253681 Free PMC article. Review.
-
The emerging role of the gut microbiota and its application in inflammatory bowel disease.Biomed Pharmacother. 2024 Oct;179:117302. doi: 10.1016/j.biopha.2024.117302. Epub 2024 Aug 19. Biomed Pharmacother. 2024. PMID: 39163678 Review.
-
A comprehensive guide to assess gut mycobiome and its role in pathogenesis and treatment of inflammatory bowel disease.Indian J Gastroenterol. 2024 Feb;43(1):112-128. doi: 10.1007/s12664-023-01510-0. Epub 2024 Feb 27. Indian J Gastroenterol. 2024. PMID: 38409485 Review.
-
Current understanding of microbiota- and dietary-therapies for treating inflammatory bowel disease.J Microbiol. 2018 Mar;56(3):189-198. doi: 10.1007/s12275-018-8049-8. Epub 2018 Feb 28. J Microbiol. 2018. PMID: 29492876 Review.
-
Links Between Inflammatory Bowel Disease and Chronic Obstructive Pulmonary Disease.Front Immunol. 2020 Sep 11;11:2144. doi: 10.3389/fimmu.2020.02144. eCollection 2020. Front Immunol. 2020. PMID: 33042125 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

