Studying the mechanisms of neurodegeneration: C. elegans advantages and opportunities
- PMID: 40207239
- PMCID: PMC11979225
- DOI: 10.3389/fncel.2025.1559151
Studying the mechanisms of neurodegeneration: C. elegans advantages and opportunities
Abstract
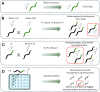
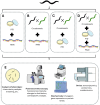
Caenorhabditis elegans has been widely used as a model organism in neurodevelopment for several decades due to its simplicity, rapid growth, short life cycle, transparency, and rather simple genetics. It has been useful in modeling neurodegenerative diseases by the heterologous expression of the major proteins that form neurodegenerative-linked aggregates such as amyloid-β peptide, tau protein, and α-synuclein, among others. Furthermore, chemical treatments as well as the existence of several interference RNA libraries, transgenic worm lines, and the possibility of generating new transgenic strains create a magnificent range of possible tools to study the signaling pathways that could confer protection against protein aggregates or, on the contrary, are playing a detrimental role. In this review, we summarize the different C. elegans models of neurodegenerative diseases with a focus on Alzheimer's and Parkinson's diseases and how genetic tools could be used to dissect the signaling pathways involved in their pathogenesis mentioning several examples. Finally, we discuss the use of pharmacological agents in C. elegans models that could help to study these disease-associated signaling pathways and the powerful combinations of experimental designs with genetic tools. This review highlights the advantages of C. elegans as a valuable intermediary between in vitro and mammalian in vivo models in the development of potential new therapies.
Keywords: C. elegans; advantages and disadvantages; genetic modulation; models; neurodegeneration; pharmacological treatment.
Copyright © 2025 Torres, Mira, Pinto and Inestrosa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

