Nanotension Relief Agent Enhances Tissue Penetration by Reducing Solid Stress in Pancreatic Ductal Adenocarcinoma via Rho/ROCK Pathway Inhibition
- PMID: 40207257
- PMCID: PMC11979343
- DOI: 10.34133/bmr.0173
Nanotension Relief Agent Enhances Tissue Penetration by Reducing Solid Stress in Pancreatic Ductal Adenocarcinoma via Rho/ROCK Pathway Inhibition
Abstract
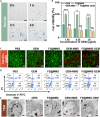
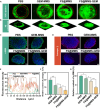
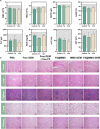
The formidable contractile tension exerted by cancer-associated fibroblasts (CAFs) in pancreatic ductal adenocarcinoma (PDAC) tissue is crucial for maintaining high tissue solid stress (TSS), which impedes the delivery and penetration of chemotherapeutic drugs. To address this obstacle, we constructed a pH-responsive nanotension relief agent (FS@MMS), in which fasudil (FS) was ingeniously conjugated to mesoporous silica encapsulated with magnetic iron oxide (MMS). The nanotension relief agent was demonstrated to inhibit the synthesis of phosphorylated myosin light chain by blocking the Rho/Rho-associated serine/threonine kinase (ROCK) pathway, triggering the swift transformation of high-tension CAFs into low-tension CAFs in PDAC tissue, which relieves TSS and enhances drug penetration in Panc02/NIH-3T3 multicellular tumor spheroids. When the nanotension relief agent was further loaded with the chemotherapeutic drug gemcitabine (GEM), as FS@MMS-GEM, the enhanced permeation of GEM progressively killed tumor cells and amplified their TSS-relief properties, thereby maximizing the anticancer efficacy of chemotherapeutic agents in Panc02/NIH-3T3 coplanted model mice. The magnetic resonance imaging results revealed that the synergistic effect substantially improved drug delivery and penetration efficiency. The developed approach holds great potential for improving chemotherapy efficacy in PDAC and provides a novel therapeutic approach for the treatment of related stroma-rich tumors.
Copyright © 2025 Feiran Yu et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures





Similar articles
-
Co-delivery of autophagy inhibitor and gemcitabine using a pH-activatable core-shell nanobomb inhibits pancreatic cancer progression and metastasis.Theranostics. 2021 Aug 4;11(18):8692-8705. doi: 10.7150/thno.60437. eCollection 2021. Theranostics. 2021. PMID: 34522207 Free PMC article.
-
Partial ligand shielding nanoparticles improve pancreatic ductal adenocarcinoma treatment via a multifunctional paradigm for tumor stroma reprogramming.Acta Biomater. 2022 Jun;145:122-134. doi: 10.1016/j.actbio.2022.03.050. Epub 2022 Apr 2. Acta Biomater. 2022. PMID: 35381402
-
Inhibition of ROCK1 kinase modulates both tumor cells and stromal fibroblasts in pancreatic cancer.PLoS One. 2017 Aug 25;12(8):e0183871. doi: 10.1371/journal.pone.0183871. eCollection 2017. PLoS One. 2017. PMID: 28841710 Free PMC article.
-
Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts.Adv Drug Deliv Rev. 2021 May;172:37-51. doi: 10.1016/j.addr.2021.02.012. Epub 2021 Mar 8. Adv Drug Deliv Rev. 2021. PMID: 33705881 Review.
-
Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: where we are and where we are going.Exp Mol Med. 2017 Dec 1;49(12):e406. doi: 10.1038/emm.2017.255. Exp Mol Med. 2017. PMID: 29611542 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Manji GA, Olive KP, Saenger YM, Oberstein P. Current and emerging therapies in metastatic pancreatic cancer. Clin Cancer Res. 2017;23(7):1670–1678. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

