Host-microbiota interactions in the infant gut revealed by daily faecal sample time series
- PMID: 40207273
- PMCID: PMC11977378
- DOI: 10.20517/mrr.2024.45
Host-microbiota interactions in the infant gut revealed by daily faecal sample time series
Abstract
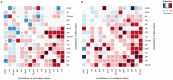
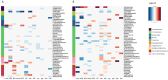
Aim: This study aims to explore the interplay between host immune factors and gut microbiota in human infants in vivo using time-series daily stool samples and identify biomarkers of host-microbe interactions. Methods: 216 faecal samples collected from infants aged 5-6 or 11-12 months were analysed for gut microbiota composition, total bacterial load, and biomarkers of immune function. Results: We identified indications of microbial stimulation of eosinophil cationic protein (ECP), IgA, calprotectin (Cal), intestinal alkaline phosphatase (IAP), and Bactericidal/permeability-increasing protein (BPI) at 6 and 12 months, as well as stimulation of lipocalin 2 (LCN2), lactoferrin (LTF), and alpha-defensin-5 only at 6 months. The associations between biomarker concentrations and bacterial population growth were primarily positive at 6 months and mostly negative at 12 months, suggesting increasing host regulation of the microbiota with age. The exceptions were IAP, which was predictive of declining bacterial populations at both time points, and Cal, whose associations changed from negative at 6 months to positive at 12 months. Conclusion: There is an age-associated development in the correlation pattern between bacterial population growth and the biomarker concentrations, suggesting that host-microbe interactions change during early development. Albumin appeared as a potential marker of gut permeability, while LCN2 seemed to correlate with gut transit time. Mucin degradation appeared to decrease with age. Mucin2 and IAP emerged as potentially important regulators of the bacterial populations in the infant gut. The study demonstrates the utility of biomarker and bacteria profiling from daily stool samples for analysing in vivo associations between the immune system and the gut microbiota and provides evidence of host regulation of the microbiota in infants.
Keywords: IAP; Infant gut microbiome; albumin; immune biomarkers; lipocalin 2; mucin.
© The Author(s) 2024.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures

Similar articles
-
The gut microbiota is associated with the small intestinal paracellular permeability and the development of the immune system in healthy children during the first two years of life.J Transl Med. 2021 Apr 28;19(1):177. doi: 10.1186/s12967-021-02839-w. J Transl Med. 2021. PMID: 33910577 Free PMC article.
-
Changes of gut microbiota and immune markers during the complementary feeding period in healthy breast-fed infants.J Pediatr Gastroenterol Nutr. 2006 May;42(5):488-95. doi: 10.1097/01.mpg.0000221907.14523.6d. J Pediatr Gastroenterol Nutr. 2006. PMID: 16707969
-
Associations between Gut Microbiota and Intestinal Inflammation, Permeability and Damage in Young Malawian Children.J Trop Pediatr. 2022 Feb 3;68(2):fmac012. doi: 10.1093/tropej/fmac012. J Trop Pediatr. 2022. PMID: 35149871 Free PMC article.
-
Microbiota-host interplay at the gut epithelial level, health and nutrition.J Anim Sci Biotechnol. 2016 Nov 8;7:66. doi: 10.1186/s40104-016-0123-7. eCollection 2016. J Anim Sci Biotechnol. 2016. PMID: 27833747 Free PMC article. Review.
-
Prebiotic effects: metabolic and health benefits.Br J Nutr. 2010 Aug;104 Suppl 2:S1-63. doi: 10.1017/S0007114510003363. Br J Nutr. 2010. PMID: 20920376 Review.
References
-
- Differding MK, Benjamin-Neelon SE, Hoyo C, Østbye T, Mueller NT. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020;20:56. doi: 10.1186/s12866-020-01723-9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
