Profiling the gut and oral microbiota of hormone receptor-positive, HER2-negative metastatic breast cancer patients receiving pembrolizumab and eribulin
- PMID: 40207279
- PMCID: PMC11977384
- DOI: 10.20517/mrr.2024.49
Profiling the gut and oral microbiota of hormone receptor-positive, HER2-negative metastatic breast cancer patients receiving pembrolizumab and eribulin
Abstract
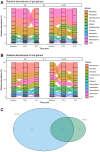
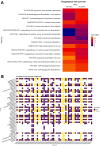
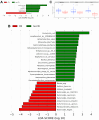
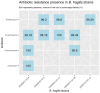
Aim: Changes in host-associated microbial communities (i.e., the microbiota) may modulate responses to checkpoint blockade immunotherapy. In the KELLY phase II study (NCT03222856), we previously demonstrated that pembrolizumab [anti-programmed cell death protein 1 (PD-1)] combined with eribulin (plus microtubule-targeting chemotherapy) showed encouraging antitumor activity in patients with hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (mBC) who had received prior treatments. Methods: A total of 58 fecal and 67 saliva samples were prospectively collected from a subset of 28 patients at baseline (BL), after three treatment cycles, and end of treatment. Shotgun metagenomics, 16S rRNA gene amplicon sequencing, and bioinformatics and statistical approaches were used to characterize fecal and oral microbiota profiles. Results: Treatment caused no substantial perturbations in gut or oral microbiota, suggesting minimal drug-related microbial toxicity. Bacteroides and Faecalibacterium were the dominant gut microbiota genera, while Prevotella and Streptococcus were present in both oral and gut samples, highlighting potential gut-oral microbial interactions. Additionally, clinical benefit (CB) appeared to be associated with gut-associated Bacteroides fragilis (B. fragilis) and a BL oral abundance of Streptococcus ≥ 30%. Notably, B. fragilis NCTC 9343 supernatant induced dose-dependent lactate dehydrogenase (LDH) release from the MCF-7 (HR-positive/HER2-negative) BC cell line. Conclusion: These findings suggest that specific gut and oral microbiota may modulate the effectiveness of combinatory anti-BC therapies, potentially through the action of microbial metabolites.
Keywords: 16S rRNA gene amplicon sequencing; Microbiota; breast cancer; eribulin; immunotherapy; metastatic breast cancer; pembrolizumab; shotgun metagenomic sequencing.
© The Author(s) 2024.
Conflict of interest statement
Robinson T received funding for conference attendance from MSD and funding from Daiichi0-Sanko for educational workshops, while the other authors have declared that they have no conflicts of interest.
Figures


References
-
- NIH. Cancer stat facts: female breast cancer subtypes. Available from: https://seer.cancer.gov/statfacts/html/breast-subtypes.html . [Last accessed on 26 Oct 2024]
-
- Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6:676–84. doi: 10.1001/jamaoncol.2019.6650. - DOI - PMC - PubMed
-
- Pérez-García JM, Llombart-Cussac A, G Cortés M, et al. Pembrolizumab plus eribulin in hormone-receptor-positive, HER2-negative, locally recurrent or metastatic breast cancer (KELLY): an open-label, multicentre, single-arm, phase II trial. Eur J Cancer. 2021;148:382–94. doi: 10.1016/j.ejca.2021.02.028. - DOI - PubMed
-
- Cortes J, O’Shaughnessy J, Loesch D, et al; EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus E7389) investigators. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 2011;377:914-23. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
