Electromechanical Regulation Underlying Protein Nanoparticle-Induced Osmotic Pressure in Neurotoxic Edema
- PMID: 40207308
- PMCID: PMC11980935
- DOI: 10.2147/IJN.S503181
Electromechanical Regulation Underlying Protein Nanoparticle-Induced Osmotic Pressure in Neurotoxic Edema
Abstract
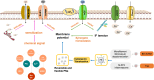
Purpose: Osmotic imbalance is a critical driving force of cerebral edema. Protein nanoparticles (PNs) amplify intracellular osmotic effects by regulating membrane potential and homeostasis of water and multiple ions. This study has investigated how PNs control the neuronal swelling through electromechanical activity.
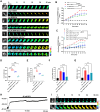
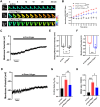
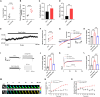
Methods: The fluorescence resonance energy transfer (FRET)-based Vimentin force probe was used to real-time monitor the osmotic tension in neurons. Patch clamp and the living cell 3D imaging system were applied to explore the relationship between cell electromechanical activity and cell volume in different cytotoxic cell models. Cytoplasmic PN amount measured by the NanoSight instrument, ion contents detected by the freezing point osmometer and ion imaging were performed to investigate the role of PNs in regulating cell swelling.
Results: We observed a close association between neuronal swelling and changes in osmotic tension and membrane potential. The tension effect of biological osmotic pressure (OP) relies on electromechanical cooperation induced by intracellular PN and Ca2+ levels. PNs increment results from cytoplasmic translocation of intracellular various proteins. Alterations in Ca2+ content are involved in the membrane potential transition between depolarization and hyperpolarization in a PN-dependent manner. Chemical signals-mediated sensitization of ion channels has an indispensable effect on PN-induced ion increments. Notably, aquaporin-mediated water influx recovers membrane potential and enhances osmotic tension controlling neuronal swelling.
Conclusion: Our findings indicate that PNs, Ca2+, and water are pivotal in electromechanical cooperation and provide insights into the biological OP mechanisms underlying neurotoxic edema.
Keywords: electromechanical cooperation; membrane potential; neuronal swelling; osmotic tension; protein nanoparticle.
© 2025 Zheng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Intracellular ion and protein nanoparticle-induced osmotic pressure modify astrocyte swelling and brain edema in response to glutamate stimuli.Redox Biol. 2019 Feb;21:101112. doi: 10.1016/j.redox.2019.101112. Epub 2019 Jan 14. Redox Biol. 2019. PMID: 30685709 Free PMC article.
-
Protein Nanoparticle-Related Osmotic Pressure Modifies Nonselective Permeability of the Blood-Brain Barrier by Increasing Membrane Fluidity.Int J Nanomedicine. 2021 Mar 1;16:1663-1680. doi: 10.2147/IJN.S291286. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33688184 Free PMC article.
-
Co-activation of NMDAR and mGluRs controls protein nanoparticle-induced osmotic pressure in neurotoxic edema.Biomed Pharmacother. 2023 Dec 31;169:115917. doi: 10.1016/j.biopha.2023.115917. Epub 2023 Nov 25. Biomed Pharmacother. 2023. PMID: 38006617
-
Mechanosensory ion channels in charophyte cells: the response to touch and salinity stress.Eur Biophys J. 2002 Sep;31(5):341-55. doi: 10.1007/s00249-002-0222-6. Epub 2002 Jun 13. Eur Biophys J. 2002. PMID: 12202910 Review.
-
Impact of cell swelling on proliferative signal transduction in the liver.J Cell Biochem. 2001 Jun 26-Jul 25;83(1):56-69. doi: 10.1002/jcb.1205. J Cell Biochem. 2001. PMID: 11500954 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

