Pharmacovigilance study of immunomodulatory drug-related adverse events using spontaneous reporting system databases
- PMID: 40207612
- PMCID: PMC12032464
- DOI: 10.1177/03946320251327618
Pharmacovigilance study of immunomodulatory drug-related adverse events using spontaneous reporting system databases
Abstract
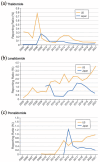
The aim of this study was to evaluate the country-specific reporting status profile of immunomodulatory drugs (IMiDs)-related adverse events (ImrAEs) in real-world clinical practice, using data from the Japanese Adverse Drug Event Report (JADER) and Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) databases. Immunomodulatory drugs, including thalidomide and its derivatives, are a new class of anticancer and anti-inflammatory drugs. IMiD risk management programs have instituted sufficient measures to prevent fetal effects but do not address adverse effects experienced by patients themselves. To date, no study has compared ImrAE profiles across countries. Adverse events were defined using the preferred terms in the Medical Dictionary for Regulatory Activities. The number of reported adverse events related to IMiDs in each country (the United States and Japan) was investigated. In both Japan and the United States, myelosuppression, pneumonia, and neuropathy peripheral have been reported as adverse events suspected to be associated with IMiDs. Adverse event profiles differed between the countries. The number of adverse event reports for thalidomide increased transiently in the United States in 2008 following the multiple myeloma indication, and then exhibited a downward trend. The number of adverse event reports for lenalidomide and pomalidomide has increased in the United States since their launch. The number of transient reports increased in Japan in 2015, when pomalidomide was launched. In this study, the profile of ImrAEs was revealed using the FAERS and JADER databases. Our comparative safety study indicated the importance of comparing the safety profiles of IMiDs using post-marketing real-world data. It is important to focus on the adverse events experienced by patients taking IMiDs, as well as the effects of IMiDs on fetuses.
Keywords: FDA adverse event reporting system database; Japanese adverse drug event report database; immunomodulatory drug; lenalidomide; pomalidomide; thalidomide.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
Post-marketing safety of immunomodulatory drugs in multiple myeloma: A pharmacovigilance investigation based on the FDA adverse event reporting system.Front Pharmacol. 2022 Dec 1;13:989032. doi: 10.3389/fphar.2022.989032. eCollection 2022. Front Pharmacol. 2022. PMID: 36532784 Free PMC article.
-
Signal mining and analysis of adverse events of Brentuximab Vedotin base on FAERS and JADER databases.PLoS One. 2025 May 16;20(5):e0322378. doi: 10.1371/journal.pone.0322378. eCollection 2025. PLoS One. 2025. PMID: 40378127 Free PMC article.
-
Post-marketing safety profile of ganirelix in women: a 20-year pharmacovigilance analysis of global adverse drug event databases (2004-2024).BMC Pharmacol Toxicol. 2025 Apr 22;26(1):91. doi: 10.1186/s40360-025-00920-4. BMC Pharmacol Toxicol. 2025. PMID: 40264185 Free PMC article.
-
Interstitial lung disease with antibody-drug conjugates: a real-world pharmacovigilance study based on the FAERS database during the period 2014-2023.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241299935. doi: 10.1177/17534666241299935. Ther Adv Respir Dis. 2024. PMID: 39660786 Free PMC article.
-
Romosozumab adverse event profile: a pharmacovigilance analysis based on the FDA Adverse Event Reporting System (FAERS) from 2019 to 2023.Aging Clin Exp Res. 2025 Jan 14;37(1):23. doi: 10.1007/s40520-024-02921-5. Aging Clin Exp Res. 2025. PMID: 39808360 Free PMC article.
References
-
- Takagi T, Bennekom CV, Amann S, et al. (2015) Risk management of teratogenic drugs ~The current states of practice in Europe, US and Japan~. Saikikeisei no aru iyakuhin no risukumanejiment ~Oushu, beikoku no genjou to nihon no mezasumichi~. Article in Japanese. Yakugaku Zasshi 135(10): 1161–1168. DOI: 10.1248/yakushi.15-00118. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

