High-throughput screen of 100 000 small molecules in C9ORF72 ALS neurons identifies spliceosome modulators that mobilize G4C2 repeat RNA into nuclear export and repeat associated non-canonical translation
- PMID: 40207633
- PMCID: PMC11983130
- DOI: 10.1093/nar/gkaf253
High-throughput screen of 100 000 small molecules in C9ORF72 ALS neurons identifies spliceosome modulators that mobilize G4C2 repeat RNA into nuclear export and repeat associated non-canonical translation
Abstract
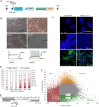
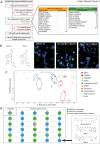
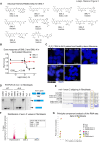
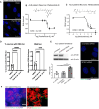
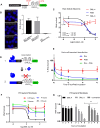
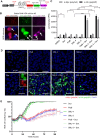
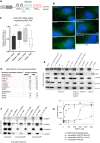
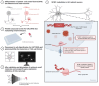
An intronic G4C2 repeat expansion in the C9ORF72 gene is the major known cause for Amyotrophic Lateral Sclerosis (ALS), with current evidence for both, loss of function and pathological gain of function disease mechanisms. We screened 96 200 small molecules in C9ORF72 patient iPS neurons for modulation of nuclear G4C2 RNA foci and identified 82 validated hits, including the Brd4 inhibitor JQ1 as well as novel analogs of Spliceostatin-A, a known modulator of SF3B1, the branch point binding protein of the U2-snRNP. Spliceosome modulation by these SF3B1 targeted compounds recruits SRSF1 to nuclear G4C2 RNA, mobilizing it from RNA foci into nucleocytoplasmic export. This leads to increased repeat-associated non-canonical (RAN) translation and ultimately, enhanced cell toxicity. Our data (i) provide a new pharmacological entry point with novel as well as known, publicly available tool compounds for dissection of C9ORF72 pathobiology in C9ORF72 ALS models, (ii) allowing to differentially modulate RNA foci versus RAN translation, and (iii) suggest that therapeutic RNA foci elimination strategies warrant caution due to a potential storage function, counteracting translation into toxic dipeptide repeat polyproteins. Instead, our data support modulation of nuclear export via SRSF1 or SR protein kinases as possible targets for future pharmacological drug discovery.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
The following authors are or were employees of the Novartis Institutes of Biomedical Research at the time this work was performed: Maartje Luteijn, Dominic Trojer, Hicham Mahboubi, Nicolas Pizzato, Martin Pfeifer, Hans Voshol, Elisa Giorgetti, Carole Manneville, Isabelle P.M. Garnier, Matthias Müller, Fanning Zeng, Kathrin Buntin, Roger Markwalder, Harald Schröder, Jan Weiler, Dora Khar, Tim Schuhmann, Paul J. Groot-Kormelink, Caroline Gubser Keller, Pierre Farmer, Angela MacKay, Martin Beibel, Guglielmo Roma, Giovanni D’Ario, Claudia Merkl, Michael Schebesta, Marc Hild, Fiona Elwood, Mark Labow, Daniela Gabriel, Mark Nash1, Jürg Hunziker and Nicole C. Meisner-Kober.
Figures

References
-
- Wales S, Kiernan DSc AM C, Cheah MBiostat BC et al. Seminar amyotrophic lateral sclerosis. Lancet. 2011; 377:423–33.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

